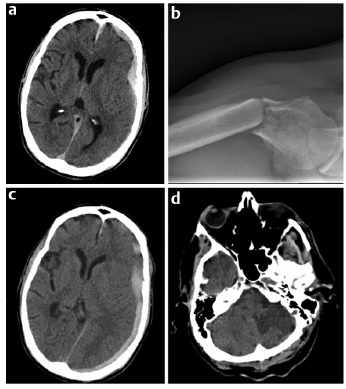
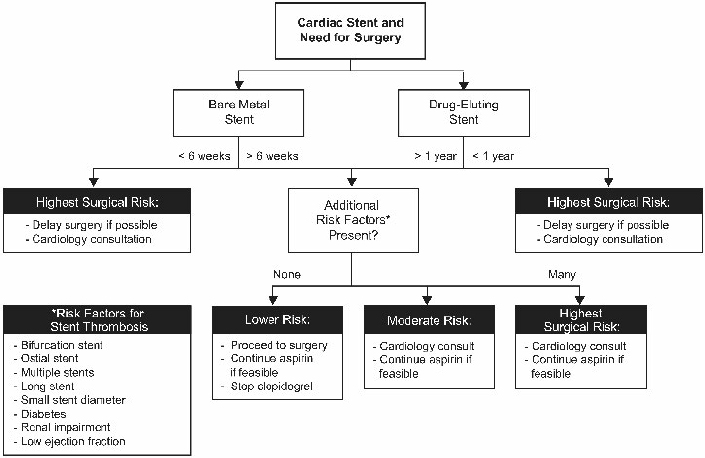
12 Arterial and venous thrombotic events combined, which include acute coronary syndromes, stroke, peripheral arterial thrombosis, deep venous thrombosis, and pulmonary embolism, are likely responsible for more morbidity and mortality than any other condition in the developed world. A common feature of the management of all thromboembolic vascular diseases is the use of antithrombotic agents. These agents, which include antiplatelet drugs, anticoagulants, and thrombolytic agents, are used to prevent thrombotic events, prevent or mitigate the complications of thrombotic events, and restore vascular patency to prevent loss of tissue, organ, and limb function. Although lifesaving for many patients, these antithrombotic therapies present unique challenges for managing patients with neurosurgical problems taking antithrombotic medications. In particular, the management of neurosurgical patients taking antiplatelet agents for coronary artery disease or on anticoagulation protocols for atrial fibrillation or mechanical heart valves presents complex risk/benefit dilemmas. Further, the introduction of novel oral anticoagulants into clinical use during the past few years adds additional complexity. An estimated 40% of United States adults take an antiplatelet agent for the prevention of cardiovascular or cerebrovascular disease. Multiple studies have demonstrated the benefits of antiplatelet agents in preventing cardiovascular disease.1 Patients on primary prophylaxis (no known cardiovascular disease), on secondary prophylaxis (known cardiovascular disease), or who have a cardiac stent have inherently different risks associated with temporary cessation of antiplatelet therapy.1 Studies have shown that in the setting of primary prophylaxis, antiplatelet drugs provide benefits against myocardial infarction in men and stroke in women, but are associated with increased risks (e.g., ulcers/gastrointestinal bleeding and hemorrhagic strokes).2 Because of these known risks, antiplatelet drugs are recommended for primary prophylaxis only for patients with a demonstrable increase in cardiovascular risk.1 The United States Preventative Task Force recommends the use of aspirin to reduce myocardial infarction in men (45–80 years old) and stroke in women (55–80 years old) when the potential benefit in each group outweighs the risk of gastrointestinal hemorrhage (http://www.uspreventiveservicestaskforce.org/uspstf/uspsasmi.htm). In contrast, studies on secondary prophylaxis show that antiplatelet drugs are superior to anticoagulation medications for recurrent myocardial infarction and superior to placebo for the prevention of myocardial infarction, sudden death, and the progression of coronary artery disease in patients with stable angina.1 Although patients appropriately prescribed antiplatelet therapy for primary or secondary prevention of cardiovascular disease gain net benefit, the benefits are small enough that when faced with a surgery with high bleeding risk, such as most neurosurgical procedures, cautious perioperative management is warranted. Because the risk of thrombotic complication is low, the perioperative recommendation for patients prescribed an antiplatelet agent for primary prophylaxis is to stop antiplatelet medications 5 to 7 days before surgery and resume them once the postoperative window for increased hemorrhage is closed.1 In contrast, one meta-analysis showed that if patients on aspirin for secondary prophylaxis routinely stopped taking it for surgery, they were at a significantly increased risk for cardiovascular events.1,3 This risk must be weighed against evidence that concurrent aspirin use can cause a 50% increase in significant hemorrhage during the perioperative period, which is particularly problematic for the neurosurgical patient.4,5 Therefore, the perioperative recommendation for patients taking antiplatelet drugs for secondary prophylaxis in the setting of a surgery with high bleeding risk is drug cessation 5 to 7 days before surgery and resumption 24 hours after surgery (or whenever the surgeon deems the risk of hemorrhage in the particular patient to be low enough).1 If a patient is taking antiplatelet medication and urgent or emergent surgery is required, then the 2012 American College of Chest Physicians (ACCP) recommendation is to “transfuse platelets or other hemostatic agents” to try to prevent excessive intraoperative bleeding.6 The most difficult clinical scenario encountered by neurosurgeons relating to antithrombotic medications is when patients with recently placed cardiac stents or recent acute myocardial infarction need neurosurgical interventions. With more than 6 million patients having cardiac stents and 5% of these patients needing surgery within 1 year of stent placement, all surgeons must know the risk of antiplatelet cessation in these patients to better educate patients and their families about the high risks involved.7–9 A prothrombotic milieu, which lasts 1.5 to 3 months with bare metal stents and 12 months in drug-eluting stents, is created after stent placement, and dual antiplatelet therapy (concomitant aspirin and thienopyridine) significantly reduces the risk of cardiovascular events when compared with aspirin alone.7–9 Furthermore, because of a high risk of recurrent ischemia, 1 year of dual antiplatelet therapy is also recommended for patients who do not receive a cardiac stent after acute coronary syndrome or myocardial infarction.1 The statistics behind the risk of antiplatelet cessation in patients with recently placed stents are alarming: antiplatelet drug cessation increases the cardiac complication rate with an odds ratio of 89.8.3,8 Furthermore, the most significant independent risk factor for stent thrombosis is premature antiplatelet cessation (odds ratio 14–57), and 30 to 40% of the instances of antiplatelet cessation were for surgery.1,8,10 The adverse cardiac event rate in patients undergoing surgery who temporarily cease antiplatelet therapy is more than double if this occurs < 30 days after stent placement than if patients are > 90 days from stent placement.7 More sobering evidence is provided by studies that showed that interruption of dual antiplatelet therapy in the first month after placement of a stent for noncardiac surgery led to a cardiac mortality rate of 86%, whereas the mortality rate was just 5% in patients who maintained dual antiplatelet therapy, and that with drug-eluting stents, the stent thrombosis rate was 31% in patients who stopped taking thienopyridine and clopidogrel and 0% in patients who did not stop.8 Yet, when dual antiplatelet therapy is continued through the perioperative period, the relative bleeding risk is increased 50% when compared with use of aspirin alone, and there is an increase in blood transfusion requirements.4–6 Because of this very complex risk/benefit situation, the perioperative management of the neurosurgical patient with a cardiac stent should be done systematically with three main considerations. First, the patient’s cardiologist should be involved in management decisions. Second, surgery should be delayed, if possible, until the very high prothrombotic state after stent implantation is lessened: 1 year for patients with a drug-eluting stent and 3 months for those with a bare metal stent. Third, aspirin therapy should be maintained whenever possible in these patients who are at a high risk for a perioperative cardiovascular event unless the bleeding risk of the surgery prohibits doing so.9 Case example 1 provides a clinical scenario that highlights the difficulty of managing a patient with traumatic intracranial hemorrhage and recent placement of cardiac stents. An 83-year-old man with a past medical history of coronary disease with previous multivessel coronary artery bypass grafting, diabetes, hypertension, and prostate cancer presented with a non–ST-segment elevation myocardial infarction (nonSTEMI) and had three drug-eluting stents placed as treatment. After the procedure, he fell and became slightly confused (not oriented to location), but had a Glasgow Coma Scale score of 14 and was otherwise neurologically intact. A computed tomography (CT) scan showed a 1-cm left acute subdural hematoma with 5 mm of midline shift (Fig. 12.1a). He also suffered a right humeral head fracture as result of his fall (Fig. 12.1b). After the patient was transferred from the medical intensive care unit to the neurological intensive care unit, a repeat CT scan was stable. The patient’s aspirin therapy regimen was continued, but clopidogrel was discontinued in case he suffered a neurologic decline and needed urgent subdural hematoma evacuation. Although the patient was monitored with serial electrocardiograms every 8 hours because of the high risk of stent thrombosis, he developed chest pain, and a subsequent echocardiogram demonstrated a new wall motion abnormality consistent with myocardial infarction from stent thrombosis. Serial CT scans every 3 to 4 days showed the subdural hematoma was stable (Fig. 12.1c). After the patient had developed a slight facial droop on hospital day 7, another CT scan showed the patient had suffered a left subacute posterior inferior cerebellar artery stroke (Fig. 12.1d). On hospital day 13, the patient had an open reduction and internal fixation of his right humerus but suffered a massive STEMI and did not survive. A recent survey of interventional cardiologists further demonstrates the complexity and uncertainty in managing these patients. Forty-eight percent of respondents said that if their patient required surgery, they would find a surgeon who would be willing to operate while the patient continued taking dual antiplatelet therapy. On the other hand, 50% said they would use an intravenous platelet glycoprotein IIb/IIIa inhibitor as a bridge during the perioperative period (although there is no controlled evidence to support this practice, especially in intracranial surgery patients who are at high risk for hemorrhage).11 When asked about patients who had drug-eluting stents for more than 1 year but needed surgery, 48% of interventional cardiologists still said aspirin therapy should be continued, 41% said dual antiplatelet therapy was necessary, and only 11% said they could be stopped.11 The ultimate decision in these situations needs to be made by the surgeon as part of a multidisciplinary approach with the cardiologists and thrombosis specialists, and the patient and family must be made aware of the high risks associated with each approach. Fig. 12.2 provides a summary of perioperative antiplatelet management considerations in these patients. Fig. 12.1a–d Case example 1. (a) Initial head computed tomography (CT) showing 1-cm left acute subdural hematoma with 5-mm midline shift. (b) Right anteroposterior shoulder radiograph demonstrating humeral head fracture. (c) CT scan from several days after initial trauma showing stable evolution of acute subdural hematoma. (d) Head CT showing subacute left posterior inferior cerebellar artery stroke on hospital day 7 after patient developed facial droop. Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and is the underlying cause of 100,000 strokes annually in the United States. Furthermore, AF-related strokes result in a 25% mortality rate and 35% severe disability rate in survivors.2,12–14 Because AF is so common and has an increased risk of stroke, it is the most common indication for anticoagulation with vitamin K antagonists (VKA); 1 million people in the United States take warfarin for AF each year, and by 2050 the worldwide estimated use will increase from 6 million to 10 million people.15 Fig. 12.2 Risk stratification and treatment algorithm for patients with cardiac stents who need surgery. (Adapted from Vinik et al4 and Riddell et al.38) Atrial fibrillation has been shown to be an independent risk factor for mortality because it increases the risk of stroke, negatively affects the heart, can worsen heart failure, and can lead to increased mortality in patients with myocardial infarction.16 The overall annual risk of stroke for all AF patients without antithrombotic drugs is 5%. Importantly, the annual risk of stroke for AF patients is not the same for all patients—a consideration that has important implications for perioperative management. An individual patient’s stroke risk can be estimated based on the number of associated clinical risk factors. A commonly used risk stratification scheme, the CHADS2 score, is shown in Table 12.1. By applying the CHADS2 score, patients can be categorized as high, medium, and low risk for stroke (annual stroke risks of < 4%, 4–10%, and > 10%, respectively).4,6 Most patients with AF should be prescribed an antithrombotic medication to reduce the risk of stroke. Warfarin is the preferred agent for most patients because it reduces the risk of stroke by > 60%16; however, aspirin, or the combination of aspirin and clopidogrel, is appropriate for some patients.16,17 Table 12.1 CHADS2 Atrial Fibrillation Stroke Risk Stratification37
Role of Anticoagulants in Common, Nonneurosurgical, Non-VTE Conditions
Cardiovascular Disease and Antiplatelet Agents
Patients with Coronary Stents
Case Example 1
Management of Patients
Anticoagulant Therapy in Patients with Atrial Fibrillation
| Points |
C = congestive heart failure | 1 |
H = hypertension | 1 |
A = age ≥ 75 years | 1 |
D = diabetes | 1 |
S2 = stroke/transient ischemic attack | 2 |
Note: Patients with scores of 0 have an annual risk of stroke of < 2%. Those with scores of ≥ 3 have an annual risk of stroke > 5% per year.
In preparation for surgical procedures, patients (i.e., all patients except those undergoing a procedure with a low risk of bleeding) must not be taking an anticoagulant because of the risk of excessive intraoperative bleeding. Thus, physicians must provide guidance as to when the use of warfarin should be stopped preoperatively, if a short-acting bridging anticoagulant (e.g., heparin or low molecular weight heparin (LMWH)) is necessary during the period of warfarin cessation, and when warfarin use should be resumed postoperatively. Simple math would suggest that because the overall stroke risk for all AF patients is 5% annually, then an 8-day perioperative period of warfarin cessation would have a risk of 0.013% daily or ~ 0.1% for 8 days.4,6 Yet, the observed risk of stroke/transient ischemic attack (TIA) for all AF surgical patients who have not received bridging prophylaxis may be 1% or greater.4,6 This higher than expected risk of stroke may be due to the fact that surgical patients are predisposed to thromboembolic events in the immediate postoperative period regardless of their anticoagulation status.4,6 One large study of 700 AF patients undergoing elective surgery showed an overall thromboembolic event rate of 0.6% when bridging was used in only 2.5% of patients.18
From a practical perspective, management should be individualized to the patient’s estimated risk of stroke and the bleeding risk of the surgical procedure. For low-risk AF patients (e.g., those with a CHADS2 score ≤ 2), bridging therapy is not necessary.4 In these patients, warfarin therapy should be stopped 5 to 6 days before surgery, and the international normalized ratio (INR) should be checked 24 hours before surgery to determine whether any reversal agents are necessary.4 If the INR is not at the goal of < 1.3, then 2.5 mg of oral vitamin K should be administered to ensure that the INR is at the goal the morning of surgery. In patients at moderate or high risk of AF (e.g., those with a CHADS2 score of 3–4 and 5–6, respectively; patients with a recent cerebrovascular accident [CVA]/TIA or rheumatic valvular heart disease are also high risk), warfarin should be stopped 5 to 6 days preoperatively, and the decision to use bridging therapy with heparin or LMWH must be made on a case-by-case basis. For most patients, bridging therapy can be used safely in the preoperative period but should be used cautiously in the postoperative setting in which bleeding risk is heightened; the risk of severe hemorrhage after major surgery can be as high as 20% when full-dose bridging is used.4,19
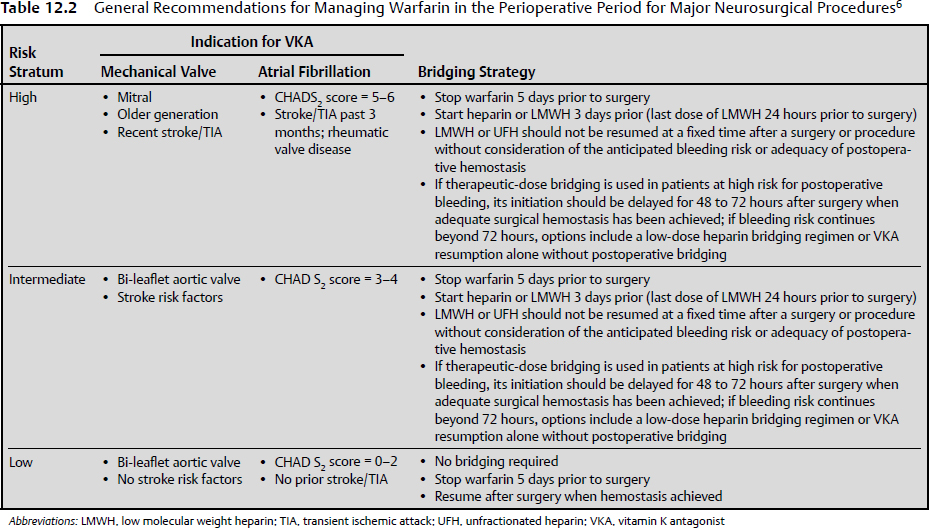
The 2012 ACCP recommendations favor LMWH over a heparin infusion as a bridge therapy because the costs are equivalent and LMWH has demonstrated noninferiority, is much easier to administer, and does not require laboratory monitoring.6 If LMWH is used, then the last dose should be half of the daily dose given > 24 hours before surgery. If intravenous heparin is used, it should be stopped 4 to 6 hours before surgery.6 The timing of postoperative resumption of anticoagulants must be determined by the surgeon and be based on the patient’s individual risk level. The postoperative hemorrhage rate for all surgery types is doubled when prophylactic doses of heparin are begun at 4 to 8 hours rather than 12 to 24 hours postoperatively.6,20 Furthermore, if therapeutic heparin is resumed within 24 hours of surgery, the odds ratio for major hemorrhage is 4.8, and the major hemorrhage rate after resumption of therapeutic heparin after major surgery is 10 to 20%.4,6,20 The risk of postoperative hemorrhage also increases with increasing patient age.21 When patients are at a high risk for hemorrhage postoperatively, it is recommended to wait at least 48 to 72 hours to resume therapeutic anticoagulation or to use low dosages or avoid parenteral anticoagulation entirely.4,6 Once again, the decision must be made on a case-by-case basis by evaluating the patient’s probability of hemorrhage and the risk of thromboembolic event.4 Table 12.2 summarizes a perioperative approach to the patient on long-term anticoagulant therapy.
Anticoagulant Therapy in Patients with Mechanical Heart Valves
Heart valve replacement surgery has been a lifesaving intervention for 50,000 to 100,000 patients a year for over 50 years, with > 80 different heart valve models used since 1950 (http://emedicine.medscape.com/article/780702-overview). Heart valve replacement is one of the most common indications for anticoagulation therapy to prevent the risk of CVA and other systemic thromboembolic conditions.
Perioperative Management
The management of the duration and timing of the cessation of anticoagulation therapy in the perioperative setting in patients with mechanical heart valves undergoing additional surgery is similar to the strategy used for AF patients with respect to the need for stratification based on risk (Table 12.2).4,6 Low-risk patients include those who have a bi-leaflet aortic valve prosthesis without AF and have no other risk factors for stroke such as prior CVA/TIA, hypertension, diabetes mellitus, congestive heart failure, or age > 75 years. These patients probably do not need bridging therapy, but low-dose subcutaneous LMWH could be used if bridging therapy was desired.4,6 Moderate-risk patients are those who have a bi-leaflet aortic valve prosthesis but also have one of the risk factors for CVA.4,6 Recommendations for this group are the same as those for moderate-risk AF patients with a CHADS2 score of 3 to 4; decisions on anticoagulation therapy must be made on an individual basis. The risk of potential hemorrhage after major surgery is as high as 10 to 20% in patients who restart therapeutic heparin within 24 hours, which has led to the official recommendation that therapeutic anticoagulation be delayed at least 48 to 72 hours (if it is resumed at all).6 The same recommendations hold true for high-risk heart valve patients (those with any mitral valve prosthesis or an older type of aortic valve prosthesis [e.g., tilting disk or caged-ball] and those with a recent CVA/TIA).6 If the decision to use bridging therapy is made, then the same drug choice, timing, and dosing parameters used for AF patients apply as discussed earlier in the AF section.6
The Emergence of New Pharmacological Antithrombotic Agents
The complexity of managing surgical patients taking antithrombotic medications has become much more difficult because of the development of newer anticoagulant and antiplatelet drugs that can be more potent and generally lack an antidote. An exhaustive description of the agents is beyond the scope of this chapter, but it is vital that practicing surgeons and critical care physicians know the basics about their indications, effectiveness, and reversibility. Major bleeding occurs annually in 1 to 2% of patients taking warfarin, including 3,500 cases of intracranial hemorrhage, and warfarin causes more deaths from adverse drug-related events each year than any other medication.15 The newer anticoagulant therapies have been developed to improve upon the many limitations of warfarin; they act faster to eliminate the need for bridging therapy, they have a more reliable and predictable anticoagulant effect to eliminate the frequent INR checks required with warfarin use, they possess a lower potential for negative dietary and other medication interactions, and they are specifically targeted to a further downfield coagulation enzyme to avoid adverse effects.22 Of emerging anticoagulants, the oral direct thrombin inhibitors and factor-Xa inhibitors have already been introduced into clinical use.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree