Fig. 5.1
COX-2 induces tumor angiogenesis via multiple pathways (Gately and Li 2004; reprint with permission from Publisher Elsevier)
COX-2 and Human Schwannomas
Schwannoma is a common tumor of the peripheral nerves and accounts for an estimated 8% of intracranial tumors. Schwannomas are considered benign tumors originating from Schwann cells with a growth rate of about 1–2 mm/year. The average proliferative activity of schwannomas has been reported to be as low as 1–3%. Schwannomas appear sporadically in the cranium, in the spinal axis, or in peripheral nerves. The occurrence of multiple schwannomas as seen in neurofibromatosis type 2 (NF2) is usually associated with an aberration of a tumor suppressor gene on chromosome 22q12. Despite its benign histopathology, progressive growth of schwannoma may lead to a variety of neurological deficits and severe disability. With progressive growth, intracranial schwannomas may be life-threatening due to compression of the brainstem or secondary hydrocephalus.
COX-2 expression has been detected in vestibular schwannoma in different intensities (Fig. 5.2). The COX-2 expression has been localized in the cytoplasma and perinuclear regions of tumor cells (Hong et al. 2011). A significant correlation between COX-2 expression and proliferation index was demonstrated indicating the involvement of COX-2 pathways in the development and growth of schwannomas. In an early study, Kökoğlu et al. (1998) reported elevated concentration of PGE2 in schwannomas as compared to control brain tissue. Increased levels of prostaglandins TBX2, PGE2, and PGF were also detected in retroperitoneal schwannomas (Komiya et al. 1991). Prostacyclin and thromboxane are known to regulate endothelial sprouting as well as VEGF-induced vascular permeability. Nevertheless, there is no report about direct correlations between TXA2 and schwannomas until now.
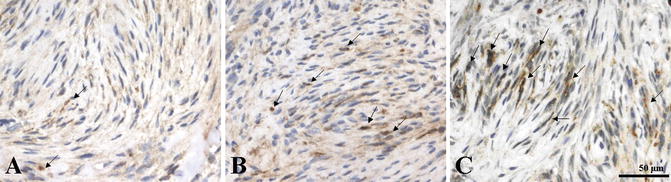

Fig. 5.2
Immunohistochemical staining of cyclooxygenase-2 (COX-2) expression in vestibular schwannomas demonstrated a diffuse or focal brown reactivity in the cytoplasma and perinuclear regions of tumor cells [arrows]. (a) Weak COX-2 expression; (b) moderate COX-2 expression; (c) strong COX-2 expression (original magnification, ×400; calibration bar 50 μm) (Hong et al. 2011; reprint with permission from Wolters Kluwer Health)
The exact role of COX-2 in development and growth of schwannomas is not known. Several pathways have been suggested which link COX-2 and growth of schwannomas. The stimulation of tumor angiogenesis through products of COX-2 resulting in increased release of numerous proangiogenic factors (e.g. vascular endothelial growth factor, VEGF; basic fibroblast growth factor, bFGF) has been suggested to be the most reasonable hypothesis. PGE2 is known to be associated with angiogenesis in tumor development by increasing various proangiogenic factors, such as VEGF, Akt, Bcl-2, αvβ3, MMP-2, MMP-9, and suppressing the production of IL-2 (Gately and Li 2004). Furthermore, numerous neurotrophic factors including nerve growth factor (NGF) (Charabi et al. 1996), TGF-ß1 (Cardillo et al. 1999), bFGF (Murphy et al. 1989), neuregulin (NRG) (Hansen and Linthicum 2004), erythropoietin (EPO) (Dillard et al. 2001), and EGF (Sturgis et al. 1996) have been suggested to have a biological role in development, maintenance, and growth of schwannomas.
It is well established, that angiogenesis is a prerequisite for proliferation and growth of any neoplasms. Even in slow-growing tumors like schwannomas, neovascularization still remains important for tumor growth. Together with MMPs and bFGF, VEGF is a potent mediator for tumor angiogenesis and vessel permeability in human schwannomas (Koutsimpelas et al. 2007). Expression of COX-2 and the resultant eicosanoid products, promote the release of VEGF. VEGF is a diffusible glycoprotein, which is expressed in Schwann cell cytoplasm. VEGF binds to its receptors, VEGR-1 and VEGR-2, which are located on vascular endothelial cells. VEGF and its receptors have been shown to promote the proliferation of endothelial cells, to increase the cells’ vascular permeability, and to induce the production of plasminogen activator (Uesaka et al. 2007).
Furthermore, VEGF supports the survival of the pre-existing tumor vasculature. More recently, VEGF has also emerged in mobilization of endothelial progenitor cells from the bone marrow to distant sites of neovascularization. Expression of VEGF in tumors of the peripheral nerve, such as neurofibroma and benign as well as malignant peripheral nerve sheath tumor (BPNST/MPNST), has been demonstrated (Wasa et al. 2008). Large tumors, recurrent tumors and tumors with a high growth rate had higher levels of VEGFR-1 (Cayè-Thomasen et al. 2005). A clinical trial with humanized monoclonal IgG1 antibody against VEGF antibody for vestibular schwannomas revealed a significantly decrease of tumor growth rate from 62% before treatment to 26% after treatment, as well as hearing improvement in most patients in selected patients with NF2-associated vestibular schwannomas (Plotkin et al. 2009). Furthermore, radiographic regression of vestibular schwannomas following anti-VEGF therapy (Bevacizumab) was described (Mautner et al. 2010).
Angiogenesis and tumor invasion require controlled degradation of the extracellular matrix (ECM) components in order to allow cell migration and new tissue formation. Tumor invasion involves always degradation of the ECM, involving enzymes such as heparinase, serine proteinases, cathepsins, and matrix metalloproteinases (MMPs). MMPs are a large family of over 20 zinc-dependent endopeptidases that proteolytically degrade most components of the extracellular matrix. MMPs are known to facilitate repair mechanisms by promoting cell differentiation and axonal growth during axonal regeneration and nerve remyelination (Lehmann et al. 2009). It was supposed, that neoplastic Schwann cells express MMPs abnormally, and degrade the ECM sheaths in the basal lamina of peripheral nerve, which is formed by collagenous matrices. MMPs are known to be able to proliferate, migrate and remodel basal lamina. The exact process, however, how Schwann cells express MMPs and invade peripheral nerve ECM remains unclear. Among the MMPs, MMP-2 and MMP-9 are most frequently involved in human tumor invasion and metastasis. MMP-2 and MMP-9 are also known to support tumor growth by neovascularization. They allow endothelial cell migration and invasion into the surrounding tissue during angiogenesis (Møller et al. 2010). Interestingly, MMP-2 and MMP-9 are expressed in diverse tumors of peripheral nerves, such as schwannoma, malignant peripheral nerve sheath tumor, and neurofibroma. In vestibular schwannoma, tumor concentration of MMP-9 correlates significantly with absolute tumor growth rate (Møller et al. 2010).
COX-2 generated prostaglandins may also enhance bFGF-induced angiogenesis through the induction of VEGF. bFGF is found in basement membranes and sub-ECM (Majima et al. 2000). Previous studies suggested that overexpression of bFGF in Schwann cells stimulates mitosis and increases the differentiation and proliferation of various neuronal populations (Grothe and Wewetzer 1996). Furthermore, bFGF has been shown to significantly increase the production of TXB2, the active metabolite of TXA2, which supports endothelial migration. In sporadic vestibular schwannomas, bFGF and VEGF correlated positively with microvascular density and tumor growth index (Koutsimpelas et al. 2007; Blair et al. 2011).
Other studies have suggested also that apoptosis resistance of tumor cells correlates with overexpression of COX-2 (Tsujii and DuBois 1995; Arico et al. 2002; Elder et al. 2002). The overexpression of COX-2 can lead to the increased production of the protein Bcl-2, which increases the survival of vascular endothelial cells. This antiapoptotic process may also support tumor growth of human schwannomas. Utermark et al. (2005) demonstrated the reduction of basal apoptosis rate of primary human schwannoma cells in comparison to that of normal Schwann cell. Finally, decreased expression of integrin αvβ3 may be involved in the changes in Schwann cell morphology, loss of extracellular matrix adhesion, and increased migration (Eliceiri and Cheresh 2006). Integrin αvβ3 and its receptors are involved in cell adhesion, migration, survival, morphology, and angiogenesis in neoplasms.
In conclusion, current data suggest that the COX-2 pathway is involved in the development and growth of human schwannomas by different mechanisms. Overexpression of COX-2 correlated with high proliferation rates of schwannomas. The increase of COX-2 products like prostaglandins resulting in stimulation of tumor proangiogenic factors is supposed to be the main mechanism. Furthermore, prostaglandin products support the release of various neurotrophic factors, which contribute additionally to tumor growth. COX-2 may present an interesting new target for medical therapy in recurrent or difficult-to-operate schwannomas. Further studies with selective COX-2 inhibitors are required to underline such concepts.
References
Bennet A (1986) The production of prostanoids in human cancers, and their implications for tumor progression. Prog Lipid Res 25:539–542CrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






