Fig. 20.1
Increased expression of MMP2 protein in the metastatic brain foci. (a) Evaluation of protein expression by Western blotting. The membranes were stripped and re-probed with β-actin antibody to confirm equal loading. (b) Quantitative analysis of MMP2 expression was determined by densitometry. The results shown in the histogram are the mean ± standard deviation from three control and three tumor samples. for statistically significant when P was ≤0.05). (c) Localization of MMP2 in the brain metastatic foci. Immunohistochemical staining (brown) of MMP2 (A, B) protein in the brain metastatic foci revealed positivity within neoplastic cell cytoplasm. Negative controls for MMP2 (C). Note that glial cells are also positive. Bars indicate 100 μm. (d) Increased expression of MMP2 mRNA in the metastatic brain foci. Semi-quantitative RT-PCR analysis was used to detect MMP2 and β-actin in total RNAs from normal brain and metastatic brain foci. β-actin was used as an internal control
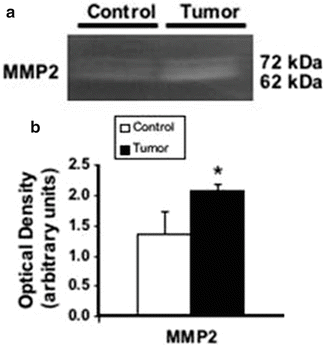
Fig. 20.2
Increased MMP2 enzymatic activities in the metastatic brain foci. (a) Evaluation of MMP2 activity by gel zymography. (b) Quantitative analysis of MMP2 activity was determined by densitometry of respective active bands (62 kDa). The results shown in the histogram are the mean ± standard deviation from three control and three tumor samples. for statistically significant when p ≤ 0.05
Matrix Metalloproteinase 2 Role in the Development of Breast Cancer Brain Metastasis
We further investigated whether MMP2 has an effect in tumor growth and metastasis development (specifically brain metastasis) in a syngeneic animal model by over-expression of TIMP2 in rat mammary adenocarcinoma -ENU1564-cells. In addition, we investigated if astrocytes are associated with BC brain metastasis development and MMP2 expression, and if this association has a relationship with MAPK pathway components.
Because we did not observe development of brain metastases (Fig. 20.3a) in any of the animals inoculated with ENU1564-TIMP2 cells, we used material collected from orthotopic mammary tumors developed from inoculation of both ENU1564 and ENU1564-TIMP2 cells to evaluate in vivo change in expression and activity of MMP2.
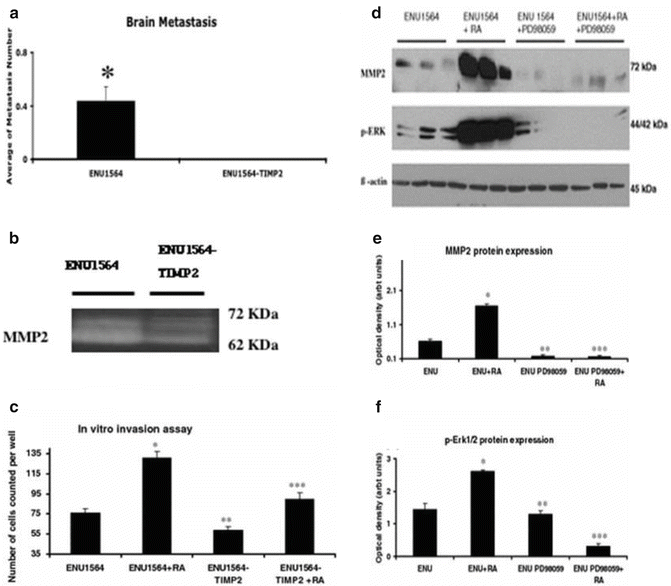

Fig. 20.3
Metastatic potential of ENU1564 vs ENU1564-TIMP2 and MMP2 enzymatic activity in orthotopic tumors originated from inoculation with ENU1564-TIMP2 vs ENU1564 control cells and ERK1/2 pathway. (a) Effects of TIMP2 overexpression in brain metastatic tumor development. for statistically significant when p < 0.05. (b) Evaluation of MMP2, activity by gel zymography. (c) In vitro invasion chamber assay for ENU1564 cells was performed and the results shown in the histogram are the mean ± SD of two individual experiments run in triplicate. RA rat astrocyte-conditioned media. Asterisk, statistically significantly different from ENU1564 (P < 0.001). (d) Evaluation of phosphorylated-ERK1/2 and MMP2 protein expression by WB. ENU15164 cells were treated with PD98059 (a potent ERK1/2-MAPK inhibitor) with or without the presence of rat astrocyte-conditioned media (RA). The membranes were striped and re-probed with β-actin antibody to confirm equal protein loading and transfer. (e) Quantitative analysis of phosphorylated-ERK1/2 expression was determined by densitometry. (f) Quantitative analysis MMP2 expression was determined by densitometry. The results shown in the histograms are the mean ± SD from three individual experiments run in triplicate. Single asterisk, statistically significantly different from ENU1564 (P < 0.05). Double asterisk, statistically significantly different from ENU1564 (P < 0.05). Triple asterisk, statistically significantly different from ENU1564-RA (rat astrocyte-conditioned media treated-ENU1564) (P < 0.001)
WB evaluation revealed non-significant difference in levels of MMP2 protein (p > 0.1). Gel zymography evaluation revealed significantly higher MMP2 activity in samples obtained from animals inoculated with ENU1564 cells when compared with animals in the control group (Fig. 20.3b). Gross evaluation did not reveal any significant changes in the central nervous system. Upon histological evaluation 44.4% of the animals in the control group had brain metastases. Brain metastases were not detected in any of the animals in the group inoculated with ENU1564-TIMP2 cells (p < 0.001).
To evaluate if TIMP2 over expression has an effect on in vivo tumor growth and metastasis development, we examined tumor progression by orthotopic inoculation of ENU1564-TIMP2 cells. We observed, at two different time points, that tumors derived from ENU1564-TIMP2 cells were significantly smaller than tumors derived from ENU1564 cells (P < 0.001 and <0.05) in the two different time points, days 32 and 42 post-inoculation. Tumor weights were obtained at the time of sacrifice (day 42 post-inoculation). Additionally, post-mortem evaluation revealed that only animals from the control group developed metastases.
Breast cancer brain metastases induce a marked astrocyte response (Nishizuka and Ishikawa 2002). We reported previously that prominent astrocyte reaction is associated with BC brain metastatic foci (Mendes et al. 2005). Therefore, we investigated if astrocytes play a role in the development of BC brain metastases in our model. To examine the astrocyte response, we evaluated GFAP (glial fibrillary protein-astrocyte marker) reactivity by IHC. We observed a marked increase of immunohistochemical staining of GFAP in reactive astrocytes around brain metastatic foci when compared with normal brain. To confirm the IHC results, we performed WB analysis for GFAP protein in samples obtained from dissected frozen brain specimens. We observed that tumor tissues express higher levels of GFAP protein when compared with control brains of animals free of tumor.
To further test the hypothesis that astrocytes have a role in MMP2 expression in ENU1564 cells, we used 48-h-rat astrocyte-conditioned media. ENU1564 cells revealed an increase in vitro invasive behavior of ENU1564 cells when compared with ENU1564 cells without astrocyte-conditioned media treatment. This invasive behavior was also reduced in ENU1564-TIMP2 transfected cells (P < 0.001) (Fig. 20.3c). Conversely, incubation with astrocyte-conditioned media increased expression of MMP2 protein when compared with MMP2 expression in ENU1564 without astrocyte conditioned media.
Our studies showed that astrocyte factors increase in vitro invasiveness of ENU1564 cells and MMP2 expression and activity are increased in BC brain metastases in our model. Since the MAPK pathway has been linked with the metastatic cascade, MMP2 activity and/or astrocyte factors we hypothesized that BC brain metastasis development and astrocyte factor-related MMP2 expression could be associated with MAPK pathway components. To test this hypothesis in our model, we performed IHC analysis for p-ERK1/2, p38, and JNK. Neoplastic epithelial cells were positive for p-ERK1/2 and staining was more intense at the periphery of the neoplastic lesions. Neoplastic cells were not stained with phosphorylated-p38 and JNK antibodies. To confirm the IHC results, we performed WB analysis for p-ERK1/2 protein in samples obtained from dissected frozen brain specimens. We observed that tumor tissue expresses higher levels of p-ERK1/2 protein when compared with control brains of tumor-free suggesting that ERK1/2 may be associated with BC brain metastasis development. Further, to evaluate the relationship between ERK1/2, MMP2 protein expression and astrocyte factors, we examined whether PD98059, a potent ERK1/2-MAPK inhibitor, can affect the expression of MMP2 in ENU1564 cells treated with 48 h astrocyte-conditioned media (Fig. 20.3d–f). We observed that p-ERK1/2 and MMP2 protein expression were increased in ENU1564 cells treated with astrocyte-conditioned media (P < 0.05) when compared with ENU1564 cells without astrocyte-conditioned media. There was no significant difference in the levels of non-phosphorilated ERK in ENU1564 cells when compared to ENU1564 cells treated with astrocyte-conditioned media. Treatment with PD98059 induced a significant decrease of MMP2 protein expression (P < 0.05) as well as the expected decrease in p-ERK1/2 expression in ENU1564 cells and ENU1564 cells treated with rat astrocyte-conditioned media (P < 0.001).
Discussion
We have determined, using a rat syngeneic model for BC brain metastases that BC brain metastases significantly express higher levels of MMP2. We also determined that there is a correlation between MMP expression and enzymatic activity within the neoplastic foci. Our results suggest that this proteinase may play a role in the development of BC brain metastases. Matrix metalloproteinase 2 is believed to play an important role in BC invasion and metastases. MMP2 over expression and activity have been associated with the invasive potential of human tumors. Active MMP2 was detected more frequently in malignant than benign breast carcinomas (Hanemaaijer et al. 2000). The tissue physiological inhibitors of MMP2 are thought to influence MMP2 activity. In human patients affected with brain cancer metastasis over-expression of MMP2 tissue inhibitor TIMP2 has been associated with poor prognosis. We observed that transfection of ENU1564 cells with TIMP2 causes in vitro TIMP2 over-expression associated with decreased in vitro invasive behavior of TIMP2-ENU1564 cancer cells. Concurrently, when these transfected cells were inoculated in vivo, there was a marked increase in TIMP2 expression. In order to characterize the effect of TIMP2 over expression in in vivo expression and activity of MMP2, we analyzed material collected from orthotopic mammary tumors developed from animals inoculated with ENU1564 and ENU1564-TIMP2 cells. As expected, no significant variation was observed in the levels of MMP2 protein expression because TIMP2 has no reported influence on MMP2 protein expression. However, tumors derived from animals inoculated with ENU1564 cells had higher MMP2 activity when compared with tumors originated from animals inoculated with ENU1564-TIMP2 cells. Additionally, TIMP2 over expression decreased in vivo orthotopic tumor growth, size, and weight; and also influenced the metastatic behavior of orthotopic tumors. None of the animals inoculated with ENU1564-TIMP2 cells developed metastases, compared with development of lung metastases in all animals inoculated with ENU1564 cells. Moreover, and in concurrence with these results, none of the animals inoculated with ENU1564-TIMP2 cells developed brain metastases. Additionally, in contrast with animals inoculated with ENU1564-TIMP1 and ENU1564-TIMP4, only animals inoculated with ENU1564-TIMP2 had statistically significant less brain metastasis than controls. Taken together, these data suggest that TIMP2 over-expression decreases the metastatic brain behavior of BC cancer cells in this model. Additionally, TIMP2 over-expression effectively decreases MMP2 activity. This suggests that MMP2 is, not only important in the development of orthotopic BC but also, in the biological brain metastatic behavior of these cancer cells. Cell transfection with TIMP2 in in vivo models decreases not only tumor growth, but also metastatic potential. Experimental TIMP over-expression relates to decreased node and pulmonary metastases in bladder cancer.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






