Fig. 1
The kinetics of Hb (a) and haptoglobin (b) levels in CSF after SAH
Our previous study had suggested intrathecal soluble CD163 shedding after SAH [6], which may partially explain the saturation of Hb–haptoglobin clearance via loss of membrane-bound CD163 sites available, if these are not replenished. We therefore proceeded to examine meningeal CD163 expression in post-mortem tissue after SAH and in control individuals. CD163 stained cells with the morphological appearance of meningeal (Fig. 2) and perivascular macrophages. SAH resulted in up-regulation of CD163 expression (Fig. 3a, two-fold, p = 0.02), in keeping with the pro-inflammatory nature of SAH. Interestingly, in meningeal areas with overlying blood, CD163 levels were lower versus areas without overlying blood (Fig. 3a, p = 0.02), approximating the levels seen in control cases. This was also observed after comparing meningeal areas with and without overlying blood from the same patients, to eliminate intra-individual variability (Fig. 3b, p = 0.03). No such difference was observed in the grey matter (ie perivascular CD163). CD163 meningeal staining is illustrated in Fig. 3.
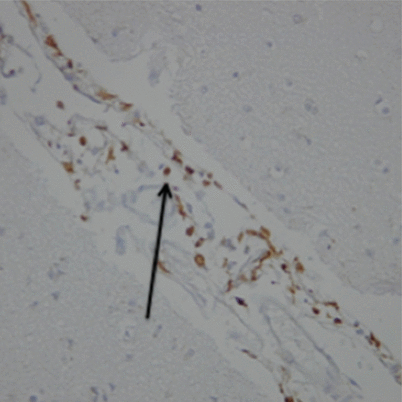
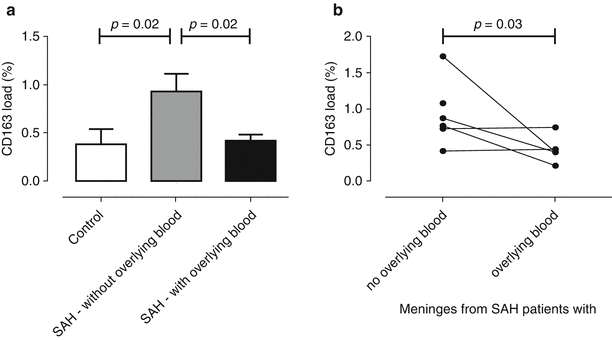

Fig. 2
CD163 immunohistochemical staining of meninges after SAH, counterstained with haemotoxylin, at magnification ×20. Arrow indicates CD163 + ve meningeal macrophages (brown)

Fig. 3
The CD163 mean percentage protein load in the meninges from control and SAH patients (a). A pairwise comparison of CD163 in SAH meninges with or without overlying blood from the same cases is shown in (b)
Discussion
In this kinetic study, we showed that CSF haptoglobin levels rise acutely after SAH as a consequence of the injection of blood into the subarachnoid space. Under normal circumstances, the CNS is deficient of haptoglobin, with the total Hb-binding capacity in the CSF being ×50,000 less than that of blood [6]. The automatic delivery of haptoglobin from serum to CSF during aneurysmal rupture may well protect against and help clear the Hb released during initial haemolysis. Haptoglobin binding to Hb prevents it from participating in toxic redox reactions [1] that damage blood vessels and neurones.
During extracranial haemolysis, haptoglobin never co-exists with free uncomplexed Hb. This is because the affinity of membrane-bound CD163 to haptoglobin–Hb complexes is very high, and the total Hb-binding capacity of the body is not limiting. We show that the situation is different during intracranial haemolysis. Not only does haptoglobin co-exist with Hb, but haptoglobin levels start rising between days 4 and 6. This is clear evidence of saturation of CD163-mediated uptake of haptoglobin–Hb complexes. Such saturation is likely to be of importance because CSF Hb levels rise further between days 4 and 6.
In this preliminary neuropathological study, we demonstrate that a potential mechanism for the persistence of haptoglobin despite high levels of Hb is the loss of membrane-bound CD163 sites available for scavenging haptoglobin–Hb complexes. Although meningeal CD163 up-regulation occurred after SAH, there was loss of CD163 in meningeal areas with overlying blood.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





