- A variety of common general medical conditions may seriously affect overnight sleep quality but not necessarily be recognised if symptoms are predominantly nocturnal and the subject sleeps alone
- Asthma, acid reflux, prostatism and chronic generalised pain syndromes are common examples
- Restless legs syndrome (RLS) is very prevalent and has a wide spectrum of severity
- Severe RLS is often not diagnosed but represents a potentially treatable cause of both insomnia and excessive daytime sleepiness
- Epilepsy or its drug treatment may produce sleep-related symptoms and poor quality sleep, which consequently may adversely affect epilepsy control
- Several rare neurological conditions or syndromes thought to reflect pathology primarily in the thalamus are associated with intractable and severe insomnia as a major clinical feature
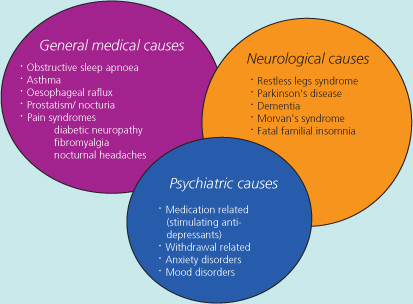
Although the term ‘secondary insomnia’ is not officially recognised by the International Classification of Sleep Disorders (ICSD-2), it has intuitive appeal and remains a useful concept in clinical practice for many. Numerous potentially reversible medical factors may disrupt both sleep onset and continuity but not necessarily be suspected as a cause of insomnia (Figure 6.1).
Figure 6.1 A simple analysis of common causes of secondary (co-morbid) insomnia. An individual may clearly have more than one ‘sleep toxin’.
Secondary insomnia is particularly common in virtually all significant psychiatric and neurodegenerative conditions (Chapters 9 and 10).
A clue that subjects have disrupted sleep resulting primarily from another medical condition is the presence of significant sleepiness during the day. This is relatively rare in chronic ‘primary’ insomnia, in which any attempts to nap after an unrefreshing or poor night’s sleep are generally unsuccessful.
The deleterious effects of increasing age on sleep quality may also interact with underlying medical problems to fuel secondary insomnia.
General medical conditions
Asthma
In large surveys almost 50% of chronic asthmatics report waking on a nightly basis with respiratory symptoms due to their underlying asthma. It may be considered so common as not to warrant mention to clinicians. In some young patients, nocturnal coughing is the only symptom related to underlying asthma.
There appears to be a significant mortality risk associated with nocturnal asthma attacks, which are frequently an indication of an imminent exacerbation.
Peak respiratory flow measurements usually drop by 10% during the night in normal subjects. This figure rises to 50% in asthmatics due to an increased nocturnal sensitivity to broncho-constricting stimuli. Lowered plasma levels of noradrenaline during sleep, increased vagal tone and altered autonomic reactivity in REM sleep may all play a role in this phenomenon.
A worsening sleep pattern with daytime lethargy may be the presenting symptoms of worsening asthma. This should prompt extra vigilance for nocturnal symptoms and potential increases in therapy.
Oesophageal reflux
Some subjects are particularly prone to oesophageal reflux of acid when lying flat at night. This propensity is worsened by spicy foods eaten late in the evening which may dilate the lower oesophageal sphincter. This can lead to sleep-onset insomnia in association with symptoms such as chest tightness, pain or acid regurgitation when reclined. However, the phenomenon may be unrecognised if it occurs during sleep and simply causes partial arousals and poor quality sleep.
There is also a clear association of acid reflux with obesity. In the presence of sleep-disordered breathing, it can therefore be difficult to determine the relative contributions of snoring, apnoea and acid-induced arousals.
Nocturnal coughing and laryngospasm producing inspiratory stridor can also arise from the adverse effects of acid reflux. A trial of an antacid treatment should be encouraged if there is clinical suspicion.
It is possible to measure nocturnal pH levels in the oesophagus with a probe. However, this is not widely available and remains a controversial area.
Nocturia and prostatism
The need to pass urine at night may result directly from a sleep disorder such as obstructive sleep apnoea but can also produce severe sleep disruption in its own right. In the elderly subject, particularly if frail or suffering from mobility problems, the act of leaving the bed may produce significant anxiety and cognitive arousal, further fuelling any insomnia.
Behavioural advice to limit fluid intake in the evening is often appropriate. In selected cases it may even be worthwhile suppressing urine production with desmopressin. In elderly males with nocturia, particularly if nocturnal confusion is a feature, the provision of a convene sheath can greatly improve the situation.
Medication to improve prostatism or bladder instability in a variety of neurological conditions may also improve sleep continuity if there is significant nocturia. However, many agents used to stabilise bladder function have pharmacological actions which may worsen other sleep-related problems. For example, anticholinergic properties may adversely influence levels of dream activity and increase limb restlessness.
Pain syndromes
Chronic pain is a common and often complex disabling symptom, affecting a wide range of subjects, both young and old. If present at night, chronic pain and the drugs used in its treatment may adversely affect both sleep quantity and quality.
There is increasing evidence from experimental and clinical populations that sleep deprivation may lower pain thresholds such that poor sleep may fuel increasing pain symptoms during the following day. This ‘bi-directional’ relationship between poor sleep and pain may even partly explain why some acute pain syndromes evolve into chronic pain when the original tissue damage has largely resolved.
Neuropathic pain
Persistent neuropathic pain is a feature of several neurological conditions. Peripheral neuropathies that particularly involve the small unmyelinated nerve fibres will typically cause painful burning or tingling, especially troublesome at night. Diabetes and alcohol-related neuropathies are common examples.
Neuralgic pain tends to be more intermittent although may interfere with sleep, especially if there is a defined cause such as shingles.
Inflammatory disorders of the central nervous system, notably multiple sclerosis, are a further recognised cause of unpleasant and arousing sensory phenomena, often at night.
Although the majority of neuropathic pain agents may cause drowsiness, their effects on sleep architecture, particularly the important deep non-REM sleep stage, are variable. It is difficult to generalise but the majority of antidepressant drugs commonly used to treat pain symptoms may worsen sleep quality overall. For example, tricyclic agents often exacerbate restless legs syndrome or cause poor sleep maintenance. Neuropathic pain agents originally developed for epilepsy, such as gabapentin and pregabalin, are usually less sleep ‘toxic’ and may even enhance the proportion of deep sleep.
Nocturnal headaches
A minority of severe headache syndromes may occur predominantly or even exclusively from sleep. Cluster headaches are often nocturnal, as are some forms of migraine. Both have been proposed to originate from REM sleep, occasionally linked to associated obstructions in breathing or even apnoeas. Apart from morning drowsiness, any resulting sleep deprivation caused by nocturnal headache may fuel further symptoms, especially in migraineurs.
Hypnic headache is a rare and poorly understood condition in which subjects awake with variable but severe pain symptoms at a particular time each night, usually between 1:00 and 2:00 a.m. It generally affects the elderly and anecdotally responds to caffeine or indomethacin before bed.
Fibromyalgia
Fibromyalgia is an extremely prevalent generalised musculoskeletal pain syndrome that affects up to 2% of the population. Precise criteria for clinical diagnosis are lacking although there is often overlap with chronic fatigue syndrome. Nocturnal sleep in fibromyalgia is usually unrefreshing in the absence of pathognomonic or specific abnormalities. When formally studied, sleep maintenance is generally poor with reduced levels of deep non-REM sleep. Non-specific phenomena may also be picked up on a polysomnogram recording. For example, a lack of sleep spindles (in light sleep) and so-called ‘alpha intrusions’ (in deep sleep) have been described as likely indicators of poor quality nocturnal sleep.
Improving nocturnal sleep in subjects with fibromyalgia can often also improve daytime pain and quality of life measures. Exercise therapy or other alternative approaches are often more successful than drugs in this respect. However, if drug therapy is considered, agents that have the least adverse effects on overnight sleep quality are more likely to be successful.
Neurological conditions
Restless legs syndrome
Restless legs syndrome (RLS) is a clinical diagnosis based on responses to four key questions (Table 6.1). Most sufferers will describe an ill-defined ache or ‘itch’ under the skin around the shins or thighs that causes them to feel restless. Rubbing or moving the affected limb produces temporary relief. Symptoms are invariably worse in the evening or when trying to sleep.
Table 6.1 Diagnosis of (restless legs syndrome) RLS depends on a positive response to four key symptoms.
| Key symptoms of RLS | Comment |
| An urge to physically move the legs usually with an ill-described discomfort in the affected limb(s) | The arms or even other body parts may be involved in severe cases |
| Symptoms worse or exclusively linked to relaxing or sitting quietly | Persistence of discomfort with activity usually indicates an alternative diagnosis |
| Rubbing or moving affected limb results in temporary relief of symptoms | Symptoms return when once again rested; other strategies include applying cold or hot stimuli to the limb or hanging the leg out of the bed |
| Symptoms invariably worse or only present in the late evening or when trying to achieve sleep | Severe cases, often on drug treatment, may report symptoms early in the afternoon |
RLS is most often an intermittent and trivial phenomenon affecting up to 5% of the population. At the severe end of the spectrum, however, subjects report severe insomnia and subsequent disabling daytime somnolence. Furthermore, associated leg movements during sleep (regular or ‘periodic’ limb jerking) may further worsen the sleep quality of severe cases as well as that of their bed partners. Active treatment is usually appropriate in those moderately or severely symptomatic on at least three evenings per week.
RLS is associated with a variety of conditions (Table 6.2) although the precise neurobiology remains obscure. There is frequently a strong familial component suggesting an autosomal dominant inheritance pattern, particularly in patients reporting symptoms before 40 years of age. Recent advances have implicated genes involved in spinal cord development as conferring particular risk for the condition.
Table 6.2 Some common associations of RLS.
| Condition associated | Comment |
| with RLS | |
| Iron deficiency | Ferritin levels should generally be checked even in the absence of anaemia; serum levels of ferritin may not correlate well with brain iron levels |
| Pregnancy | RLS very common in late pregnancy with relief after delivery of the baby |
| Renal failure | RLS is very common in chronic renal failure and often overlooked; dialysis is made particularly uncomfortable; reduced availability of iron may explain the association |
| Peripheral neuropathy | Although a controversial area, some studies have indicated subclinical neuropathy in the majority of RLS subjects |
| Depression | RLS may predispose to depression or, alternatively, be exaggerated by the majority of antidepressant drugs |
| Hypertension | Increasing evidence suggests sympathetic overactivity in RLS, with hypertension as one of the likely consequences |
Based on a variety of evidence, including specialised imaging, current theories suggest that brain iron deficiency may lie behind most forms of RLS. It is therefore important to screen also for peripheral deficiency of iron stores. If ferritin levels are below 50 mg/l, iron supplementation is recommended, even in the absence of frank anaemia.
Treatment options, when required, are listed in Table 6.3. The evidence base is most established for dopamine agonist therapy using doses generally much lower than those for Parkinson’s disease. Side effects such as nausea may limit usefulness, as may the development of augmentation. The latter refers to worsening symptoms despite an initial response to therapy. In particular, uncomfortable restlessness extends to other part of the body, such as the arms, and occurs earlier in the day than previously. At this point, alternative treatment strategies are usually indicated, since increasing dopamine agonist drug doses further generally worsens the situation.
Table 6.3 A summary of commonly used drugs for RLS. It should be noted that controlled evidence and formal licenses for prescribing exist only for the dopamine agonist drugs.
| Drug treatment for RLS | Comment |
| Dopamine agonists (DAs) | The commonly used non-ergot DAs (pramipexole, ropinirole and rotigotine) are all licensed for RLS and considered first-line therapy in moderate or severe cases; doses are generally lower than those used for Parkinson’s disease |
| Levo-DOPA | Levo-DOPA can be effective but is best used as an intermittent therapy due to rebound symptoms and augmentation occurring with prolonged courses (see text) |
| Opiates | Codeine and more powerful opiates can be effective for symptomatic control; fears over side effects and dependence limit their use |
| Anticonvulsants | Agents such as gabapentin or pregabalin can be useful as second-line (unlicensed) medications, particularly if the sensory component is prominent |
| Sedatives or hypnotics | Agents such as clonazepam may be used to improve sleep continuity non-specifically; risks of morning ‘hangover’ and increased snoring may limit usefulness |
RLS usually responds to opiate medication although it is rare to recommend long-term courses or regular treatment. However, supplemental codeine or more powerful opioid agents may be useful strategies for transient worsening of RLS symptoms. A variety of agents initially developed as anti-epileptic drugs may also have a useful role in RLS. There is most evidence for pregabalin and gabapentin, particularly if the sensory component of the RLS symptomology is prominent.
Routine hypnotics are sometimes used as a non-specific therapy in RLS to improve sleep continuity. Clonazepam in standard doses is most commonly used although it may lead to morning drowsiness.
Controlled evidence is accumulating that courses of intravenous iron therapy may help severe and resistant RLS even if there is no evidence for peripheral iron deficiency. Symptom relief can extend for months in some cases. Specialist supervision is needed to reduce the risk of anaphylactic or allergic reactions to intravenous iron by giving test doses.
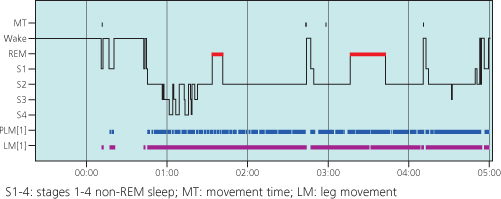
Given the symptom-based diagnosis, investigations for RLS are rarely indicated although they may reveal additional sleep pathology in drug-resistant cases. If an overnight polysomnogram is performed, however, an excess of (periodic) limb movements is usually seen, particularly in the early part of the sleep cycle, this may help to confirm the clinical diagnosis of RLS (Figure 6.2).
Figure 6.2 A hypnogram of a middle-aged male with severe RLS and associated periodic limb movements (PLMs). The trace shows a sleep-onset insomnia (due to RLS) and subsequent very frequent PLMs through the whole sleeping period. The PLMs were partially arousing the subject and preventing deep (stage 3 and 4) non-REM sleep.