Fig. 1
Diagram depicting groups of SAH patients, with poor overlap of delayed ischemic neurologic deficits (DIND), vasospasm assessed by CTA, DSA, or TCD, and delayed cerebral ischemia (DCI) consisting of clinical ischemia (or DIND) and/or infarctions seen on CT or MRI scans. Classically, deteriorating patients may be considered ischemic if we exclude new neurological signs caused by rebleed (RB), hydrocephalus (HCP), intracranial pressure (ICP) crisis, metabolic or infectious causes, anemia (Hb), hypotension (hTN), hypoxemia (hO2), seizures (SZ), etc.
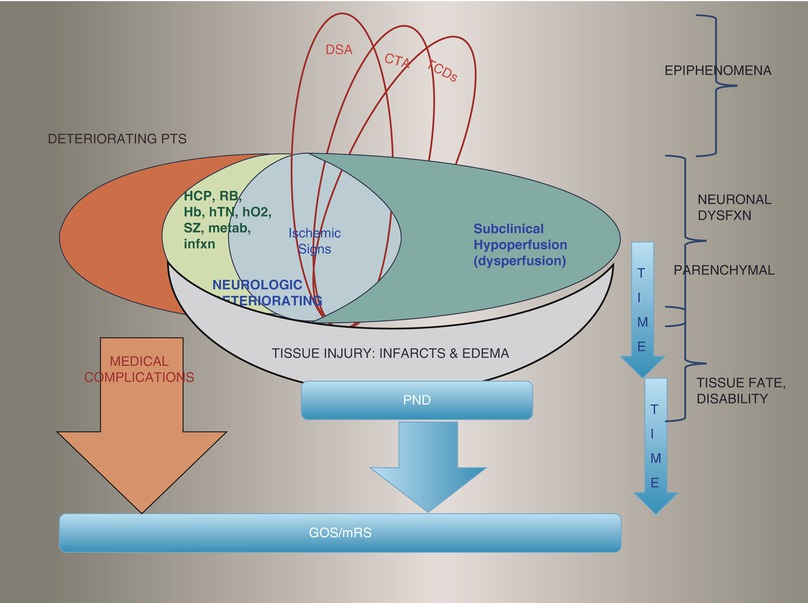
Fig. 2
Diagram depicting the pathophysiology in evolution for certain groups of worsening SAH patients. Some epiphenomena and causal processes can be detected with TCD, CTA, or DSA, or with assessment of autoregulation. However, neuronal dysfunction per se, caused by dysperfusion with ischemia or oligemia (or, on the other hand, with edema from blood-brain barrier opening) is to be measured at the parenchymal level while still ongoing, without permanent irreversible lesions. Finally, time and treatment interventions play a role. DCI, as defined recently (Frontera et al. and Vergouwen et al.) amalgamates ischemic signs on exam with permanent deficits and infarctions, whereas we prefer to keep ischemic and edematous processes at one level before the endpoint of lesion (tissue injury with completed infarctions or chronic edema). In our figure, we also point out that functional outcome (Glasgow Outcome Scale (GOS)) and disability (modified Rankin Scale (mRS)) endpoints are also greatly affected by nonpurely neurological processes such as medical complications [15]. Similar to other clinicians using DWI and CTP routinely during deterioration after SAH [10, 18, 45, 53, 70, 79, 81], we propose that perfusion imaging is the new gold standard for detection of delayed hypoperfusive injury/DCI, because subclinical hypoperfusion can be deleterious, and relying on completed infarctions to detect ischemia is dépassé. Using perfusion imaging or quantitative EEG, rather than excluding other causes of neurologic deterioration offers the advantage of eliminating the issue of concomitant processes and of silent deterioration in case of coma
PND (four RCT) is neurologic deficit upon discharge, but “persistent neurologic deficit” (usually meaning DIND lasting >2 h, or, for some others, meaning persistent deficits after hemodynamic augmentation treatment) is not interchangeable for PND. Reversibility to hyperdynamic therapy versus angioplasty is also a relevant nuance (one RCT).
Symptomatic VSP (6 RCT) is misleadingly interchanged for “clinical VSP” (6 RCT). Thirteen RCT refer to actual DIND with (10 RCT) or without excluding other causes of neurologic decline, and 9 RCT mean DIND with or without concomitant evidence of narrowed vessel but ascribed to the latter, and only 3 RCT restrict this term to only DIND with objectivated narrowed vessel. Clinical impression (2 RCT) and hierarchical diagnosis (1 RCT) are oversimplifications of DCI/VSP with the sole goal of dichotomizing patients to measure potential prognostic significance [53], whereas the subgroups are fundamentally different because they depict completely different degrees of cerebral versus vascular injuries (see the discussion below regarding DCI).
Both sonographic VSP (13 RCT) and angiographic VSP (5 RCT) have poor concordance in thresholds and in severity stratification and are poorly sensitive and specific for DCI, infarcts, or DIND [20, 21, 72].
DCI is still often referred to as probable (clinical) or definite (clinicoradiologic), but this terminology lacks descriptiveness and again, the three subgroups of DCI (clinical, radiologic, or both) are fundamentally different because these different groups of patients are suffering or suffered completely different severities of cerebral and/or vascular injuries. Very misleadingly and varying between RCT, DCI is still sometimes used to refer to the ongoing injurious ischemic process (hypoperfusive injury captured by clinical exam or perfusion imaging) or to the outcome of a completed injury with irreversible lesion (seen on imaging). Capturing ischemia as an ongoing process with mild clinical reversible manifestation cannot mean the same as completed infarctions. Also to the contrary, some infarcts may be clinically silent in good clinical grade awake patients and not portentous of major disability, whereas small lesions difficult to discern on CT scans may be responsible for major clinical deficits and disability. The amalgamated entity of DCI seems not so judicious after all, even if it is a more relevant endpoint than VSP, with higher prognostic significance [20, 21, 71, 72].
Delayed lucencies have explicit etiologic process in only 7 of the 14 RCT listed in the guidelines. Causal allocation varies from simple adjudication into three groups, to multilevel diagnostic criteria accounting for timing, location, size, number, parent vessel, and timing of clinical correlate, creating 18 types. Ictal (or acute) infarcts [57] are accounted for in only four RCT. Perfusion deficits are analyzed in only four RCT. Only one RCT went beyond a classic neurologic exam at the bedside and measured neurocognitive sequelae.
Conclusion
There is inter-RCT inconsistency in the criteria used for each subgroup of DCI and for the disparate composite endpoint of DCI [35]. Frontera et al. highlighted the clinical relevance of each subtype of DCI/VSP, putting emphasis on clinical and radiological findings being more prognostically relevant than TCD and DSA findings [21]. This was a major step forward and was embraced and verified by the community [19, 72]. However, we think that each subgroup of DCI (per Vergouwen’s definition [72]) is still intrinsically different, mixing processes and outcomes. Furthermore, recent studies scrutinizing perfusion, permeability, and vasoreactivity, as well as electrographic, spectroscopic, and intracortical probing, push the envelope of DCI even further to better delineate each category of delayed neuronal dysfunction with metabolic disarray.
Part II: Avant Garde Neuroimaging and Neuromonitoring Revolutionize Prediction, Diagnosis, and Prognosis of Delayed Cerebral Ischemia After Subarachnoid Hemorrhage
Methods
We systematically reviewed the last 20 years of English medical literature for the input of multimodal neuroimaging in understanding VSP and DCI after SAH [29, 31]. We hereby did the same to add the input of intracranial and transcranial neuromonitoring, focusing on vasoreactivity, continuous quantitative electroencephalography (EEG), cerebral oximetry (PbtO2 by Licox) flowmetry (CBF probing by Hemedex), and microdialysis (CMD), as well as near-infrared spectroscopy (NIRS). We condensed the recent qualitative and quantitative data suggesting a role for structural, sonographic, angiographic, diffusive, perfusive, dispersive, permeabilitic, and metabolic neuroimaging as well as neuromonitoring in the prediction and detection of VSP and DCI. Compiled evidence is given in the form of questions and answers.
Results
What constitutes acute brain injuries (ABI)?
Noncontrast CT [12, 57], DSA [4, 6, 47], CT perfusion (CTP) [30, 41, 44, 50, 53, 58, 70], MR perfusion (MRP) [76], DWI [24, 27, 56, 61–65, 75, 77], T2 [37], permeability [3], metabolic and spectroscopic data [25, 52, 80] colocalizing with microdialytic data [54], all together suggest cerebral ischemia/oxidative distress caused by global severe hypoperfusion [58], especially in deep watershed territories [48, 76], pial microthrombotic events [43, 51], diffuse blood-brain barrier disruption [3, 69], early global vasogenic edema [2, 3], and, rarely, ultra-early vasospasm [4].
Can we quantify ABI and correlate their severity to neurological presentation and development of DCI and also predict outcome?
Acute noncontrast CT [12, 42, 48, 57], CTP [1, 17, 41, 44, 53, 58, 70, 78, 79], MRP [23, 76, 80], DWI [57, 75–77], and T2 [37] can quantify the injury, classify patterns of infarcts [42, 48] and edema [12], and correlate with survival rates. Two Japanese studies in poor clinical grade patients use DWI to refuse aneurysmal obliteration to severely infarcted elderly patients [61–64].
Can perfusion imaging in the acute and subacute phase detect VSP/DCI earlier than clinical exam and even predict DCI?
Delayed infarcts can be predicted by the following thresholds: mean transit time (MTT) >7 s or cerebral blood flow (CBF) <34 mL/100 g/min or cerebral blood volume (CBV) < 3 mL/100 g [41] or time-to-peak (TTP) interhemispheric difference (IHD) >1.0 s [44]. Early acute perfusion imaging can predict DCI with the following thresholds: CBV <1.7 [53] or CBV interhemispheric ratio (IHR) <0.77 [70] or CBF <25 [53] or CBF IHR <0.72 [70] or MTT >5.5 s [53] or MTT IHD >0.87 s or TTP IHD >1.0 s [70].
Does the magnitude of perfusion defects correlate with the severity of angiographic VSP and impending infarction?
Perfusive defects substantiate ongoing ischemia as cause of deterioration with the following diagnostic thresholds: MTT > 5.9 s [18, 19] or 6.4 s [78] or MTT IHD >1.1 s [18, 19]. Perfusive defects substantiate significant ischemia mandating resorting to endovascular treatment with the following thresholds: MTT >7.6 s or CBF < 39. [79, 81]
What other advanced neuroimaging and neuromonitoring techniques are studied in DCI?
Loss of cerebrovascular reactivity [8] can also be detected by TCD and seems to precede DIND [22, 36] and DCI [11]. As a correlate, relative dispersion can be measured and seems to portent poorer outcome [40]. Cerebroximetric probing measures brain parenchymal oxygen tension, with low values being associated with poorer outcomes [49]. Transcranially in the bitemporal regions, NIRS can measure the cortical parenchymal amount of oxyhemoglobin, reflecting the adequacy of CBF variations. Declining values have been correlated with ischemic events and NIRS is used by some clinicians to detect DCI after SAH [8, 60].
CMD alerts clinicians to commencing metabolic distress [2], whether from ischemia, edema, or inflammation. Its sensitive detection of relative imbalance in energetic supply/demand/use has been shown to have diagnostic value during the acute and subacute phases after SAH [60, 66]. Patterns of ABI on CMD differ from those of DCI [54]. Microdialytic ischemic patterns have very good positive predictive values for both DIND and delayed infarctions [66]. Bridging CMD to clinical and neuroimaging correlates, colocalization has even been demonstrated between regions of interest with relative metabolic disarray on CMD (but without ischemia per CBF thresholds) and both relative hypoxia per positron imaging and clinical deficits in patients with DIND [55]. This corroborates earlier findings leading one to think that not all DIND patients suffer from ischemia per se; a few may experience some type of nonischemic metabolic distress, echoing the metabolic penumbra paradigm in TBI and ICH [73].
EEG: The gradual diminishment of regional cortical neuronal function (caused by ischemia, edema, inflammation, or oxidative disarray from any cause) can be detected by loss of fast frequencies and increases in slow frequencies by continuous EEG, even before visible clinical impairment. Scalp EEG seems to suffice to detect ischemic patterns with quantitative measurements such as the relative alpha variability (RAV) and alpha/delta ratio (ADR). Indeed, early detection of DCI can be performed by detecting a decrease in RAV [74] and, more recently, a drop in ADR was shown to precede DIND and infarcts after SAH by many hours [14]. Intracortical EEG, even with a single probe placed in a bundle with CMD and PbtO2, can also reveal any depreciation in neuronal function through an attenuation in electric regional power, with a significant drop in ADR a few days before documented vasospasm in ischemic SAH patients [67].
Finally, EEG is not only helpful in SAH for detecting seizures and early ischemic changes, but the presence of periodic epileptiform discharges and the absence of normal reactivity, variability, and sleep architecture are also dramatically correlated to a poorer outcome [13].
Conclusions
Robust neuroimaging data and preliminary but consistent, salient, and appealing electrographic and neuromonitoring data offer new support for diagnostic and prognostic refinements in SAH. Neurosurgeons and neurointensivists ought to become keen neurophysiologists acquainted with these new techniques and nuanced pathophysiological processes devastating their patients. Some of these modalities are already used by some groups to manage complex SAH patients [5, 8, 10, 11, 17, 28, 81]. Given the disconcerting negative results in many recent large RCT in SAH with traditional DCI/VSP definitions and endpoints, it is a necessity for our neurosurgical critical care community to devise surrogate endpoints for upcoming trials, integrating some advanced electrographic, neuroimaging, and transcranial and intracranial neuromonitoring parameters to finely depict the heterogeneity and complexity of all subgroups of deteriorating patients. Some of these parameters will surely be integrated in the common data elements efforts of the NIH and other consortiums (such as IMPACT) caring for all types of severe brain injuries [33].
Part III: Progressive Nosology for Delayed Neurovascular Insults After Aneurysmal Subarachnoid Hemorrhage
Objectives
We propose a novel classification of secondary injuries after aneurysmal SAH with better nosological terminology based on best descriptiveness of the objective clinical impairment, probed physiological disturbance, or imaged abnormality [32].
Background
We previously highlighted the terminological inconsistencies in the 38 RCT listed in the 2009 guidelines from the AHA for management of aSAH. There remains intra- and inter-RCT inconsistency in the terminology and specific criteria used for each category of DCI and VSP, even in the 2011 Neurocritical Care Society [71] and 2012 AHA guidelines [16].
Methods
Etymologic precision and rigorous adherence to purely objective description of the impairment on exam and/or abnormality documented by EEG, neuroimaging, or cerebral probing guided our new nomenclature, inspired from the RCT listed in the recent guidelines.
Results
Abandoned terms are Symptomatic VSP, Clinical VSP, DCI (probable and definite), TCD VSP, Microdialytic and EEG VSP, Ictal Infarctions, and Surgery-Related Infarctions. Remaining terms are Deterioration, DIND, Persistent Neurologic Deficit, PND, Rebleed, Hydrocephalus, Angiographic VSP, Ischemic Lesion, Perfusion Defects, Delayed Infarctions, and Retraction Injury. Proposed new terms are Semiotic as a nuance to Symptomatic, Aggravation as a nuance to Deterioration, Secondary replaces Delayed, Hypoperfusion and Relative Dispersion (RD) are nuances to Ischemia, Vasculopathy is a nuance to VSP, Hyperveloce State replaces Sonographic VSP, Loss of Flow Heterogeneity (FH) adds to Loss of Cerebral Autoregulation (or CVR), and Early Cerebral Infarctions replaces Ictal Infarcts. More importantly, hypoperfusion, edema, and oxidative disarray finally capture the entire spectrum of secondary neurologic dysfunction. Hypoperfusion and metabolic derangement now account for the ongoing injurious process, rather than relying on completed infarctions to detect ischemia [32]. Indeed, one should conceive of the evolution of post-SAH injurious processes leading to secondary injury and lesions on at least three levels (Fig. 2). Figure 3 summarizes the gross categories of patients with the specific modality to be used to detect the injurious process of each subgroup.
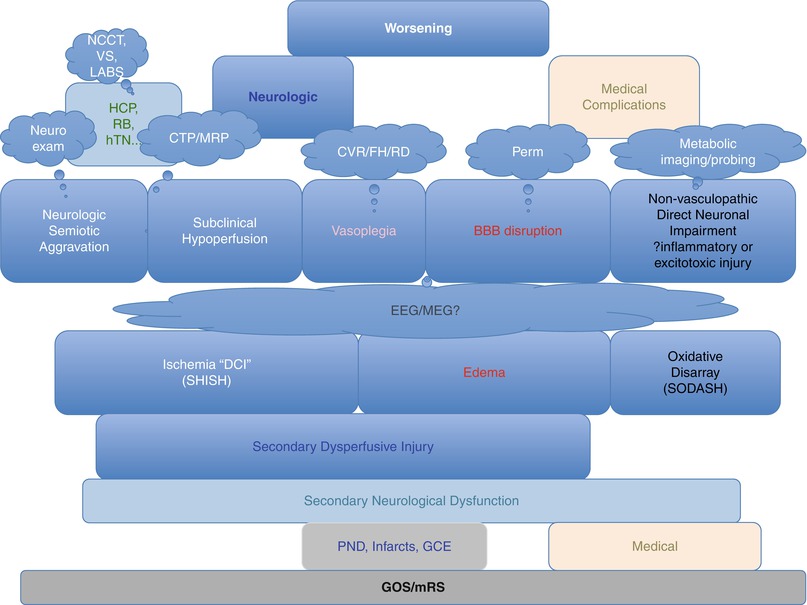

Fig. 3
Noncontrast CT (NCCT), vital signs (VS), and laboratory results allow clinicians to find obvious other causes for deterioration. Ischemic patients on clinical exam (semiotic neurological aggravation) can be aggregated with subclinical hypoperfused patients and captured all together by perfusion imaging. We suggest secondary hypoperfusive injury after SAH (SHISH) as a better term than ischemia because hypoperfusion may not always lead to overt infarction, even if sustained after SAH, as opposed to acute ischemic stroke models. Perfusion imaging also offers the possibility of measuring excessive perfusion and leakiness of the blood-brain barrier (BBB), because some patients suffer from global cerebral edema and can benefit from radically different treatment options than the classic first-line therapy consisting of hemodynamic augmentation. We also propose a distinct group for patients with metabolic derangement leading to neuronal dysfunction without any perfusive abnormality (secondary oxidative disarray after SAH (SODASH)). This group of nonvasculopathic patients may benefit the most from neuroprotective agents. Finally, cerebral infarctions and chronic edema are at a different level of endpoints. This tissue or lesional level integrates time and interventions, on the path down toward the ultimate functional endpoints, materialized into the GOS and mRS, which are affected also by medical factors
Conclusion
Finer clinical exam, TCD with pulsatility index and vasoreactivity, CMD, interstitial cerebroximetry (PbtO2) and interstitial flowmetry (CBF), NIRS, cortical and surface EEG, as well as multimodal imaging with diffusion, perfusion, relative dispersion, permeability, flow heterogeneity, positron emission, and spectroscopy shine some light onto injurious processes and hold promise for earlier detection of all types of secondary injury after SAH. Umbrella terms such as VSP and aggregated rubrics such as DCI ought to be abandoned, whereas precise terminology can describe the exact abnormality for each subtype of worsening patients. It is of utmost importance to recognize these subgroups, because of therapeutic implications. Focal versus global ischemia may not be addressed by the same therapeutic tools; edematous or intracranial hypertensive patients may not benefit from regular ischemia-targeting treatment options; loss of autoregulation and its localization might be a separate entity for a special armamentarium; and, finally, neuroprotective agents may be better suited as unique tools for the patients with no vascular impairment but with parenchymal metabolic derangement. Tailored individualized therapies may be guided by a more rigorous understanding of the specific injurious process by subgroup. A bigger impact from novel therapies may now be detectable if we use correct surrogate endpoints rather than applying global broad-spectrum multifaceted therapies and looking at an amalgamated composite endpoint of “DCI/VSP.”
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





