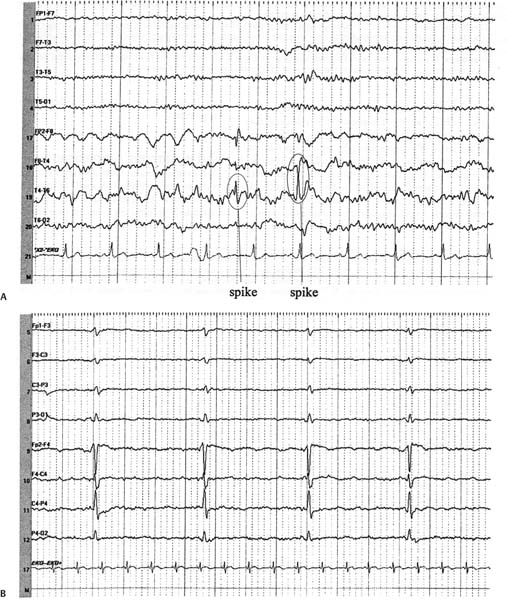
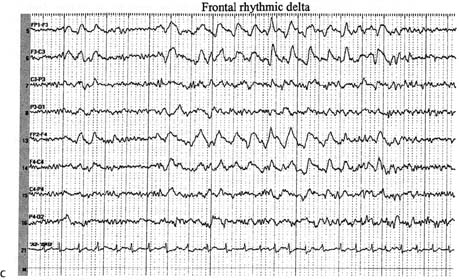
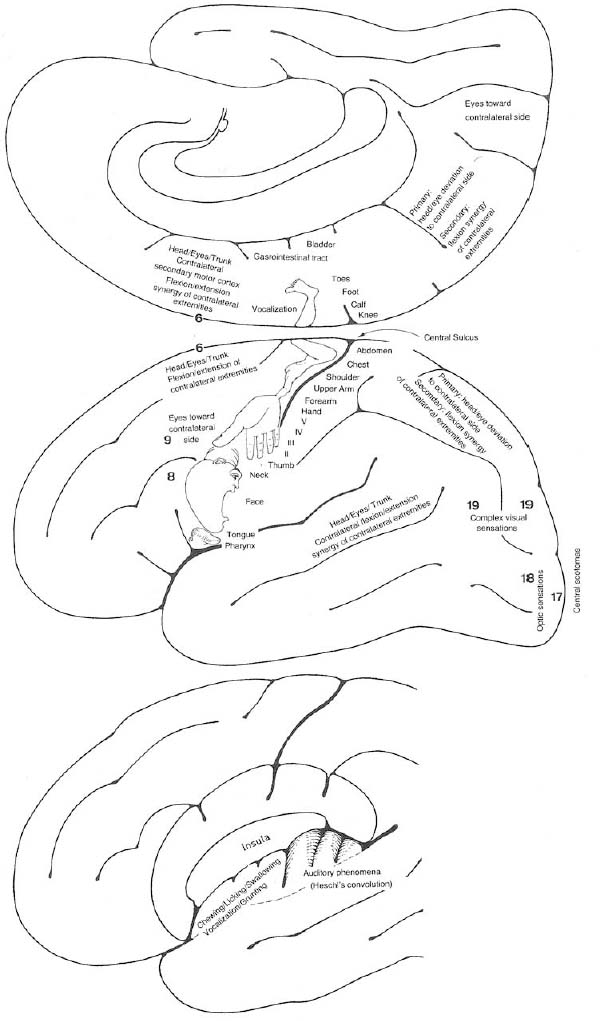
3 Note: Significant diseases are indicated in bold and syndromes in italics. 1. Scalp electroencephalogram (EEG): scalp recordings cannot detect electrical synchronization of < 6 cm2 of cortex, and have poor resolution of mesial and orbital frontal cortex, mesial parietal cortex, and mesial temporal cortex (Box 3.1) a. special electrodes i. sphenoid electrodes: inserted through temporal and masseter muscles underneath the zygomatic arch and positioned next to the foramen ovale; assesses the mesial temporal cortex ii. nasopharyngeal electrodes: inserted into the posterior nasopharynx; assesses the mesial temporal cortex (1) can also be advanced into the ethmoid sinuses to record the orbitofrontal cortex b. effects of hyperventilation: induce a clinical seizure in < 0.1% of generalized epilepsy and 0.5% of partial epilepsy, but increase interictal epileptiform discharges in 12% of generalized epilepsy and 3% of partial epilepsies; may induce ≈ 50% of absence seizures c. effects of sleep, waking, and sleep deprivation: all can provoke epilepti-form activity i. particularly good at inducing epileptiform activity in cases of infantile spasms and juvenile myoclonic epilepsy d. effects of photic stimulation: strobe light stimulation may produce i. photic driving responses: composed of rhythmic activity elicited over the posterior regions; occurs in 80% of epilepsies, but it also can be a normal physiological response ii. photoparoxysmal response: spike-and-waves or polyspike-and-waves triggered by photic stimulation in 50% of generalized seizures but only 3% of focal seizures iii. photomyoclonic response: brief repetitive spikes generated by contraction of muscles of the face or scalp that are driven by photic stimulation; seen in normal and epileptic patients e. abnormalities consistent with seizure foci (Fig. 3–1) i. sharp waves (also known as sharp-wave complexes, spikes, and spike-wave complexes) (1) occipital sharp waves are commonly seen in children and are not predictive of seizure foci (2) absence of sharp waves does not rule out seizure foci ii. amplitude asymmetries: alpha rhythm asymmetries occur normally, but generally one side is augmented rather than decreased; increased amplitudes also occur in the context of removal of portions of the skull {breech rhythm} Figure 3–1 Abnormalities consistent with a seizure focus. (A) Spike-and-wave discharge; (B) periodic lateralizing epileptiform discharges (PLEDS). (1) reduced amplitudes may occur because of cortex injury or tissue collection between the cortex surface and the electrodes (e.g., subdural hemorrhage) iii. frequency asymmetries: hemispheric background frequencies should be within 1 Hz of each other; asymmetry is a very nonspecific finding iv. polymorphic delta activity: delta activity that is variable in morphology and frequency; when present focally, it usually indicates a lesion of the white matter, but it localizes the lesion quite poorly (C) frontal intermittent rhythmic delta activity (FIRDA). (From Drazkowski J. Use of EEG in a consultative role. Semin Neurol 2000, 23: 296, Fig. 1; 300, Fig. 6; 302, Fig. 8. Reprinted by permission.) v. rhythmic delta activities: typically are associated with subcortical or even brainstem lesions when the background rhythm is otherwise normal (1) frontal intermittent rhythmic delta activity (FIRDA): occurs in adults, particularly with renal or hepatic encephalopathies or hydrocephalus (2) occipital intermittent rhythmic delta activity (OIRDA): occurs in children, particularly in the context of absence epilepsies vi. periodic lateralizing epileptiform discharges (PLEDS): focal bursts epileptiform discharges that occur repetitively every 1–5 seconds; indicates acute or subacute focal cortical injury, or occasionally a metabolic disorder (particularly nonketotic hyperglycemia) superimposed on a chronic, focal cortical injury (1) bilateral PLEDS is suggestive of diffuse cortical injury or encephalopathy 2. Prolactin level: Useful only if collected within 15–20 minutes of the seizure a. prolactin levels are increased over baseline level in 80% of generalized seizures and 70% of complex-partial seizures b. prolactin levels are rarely increased after pseudoseizures, after prefrontal seizures that symptomatically resemble the bizarre behaviors of pseudo-seizures {pseudo-pseudoseizures}, or after status epilepticus c. seizures also increase CRH and LH secretion (Box 3.2) Recurrence of Seizures: Overall risk of seizure recurrence is 40% after two years. 1. Simple partial seizures (Fig. 3–2): do not involve impairment or loss of consciousness irrespective of the complexity or distribution of convulsive activity; form the aura of complex partial seizures a. simple partial motor seizures Figure 3–2 Symptomatic manifestations of focal seizures according to the seizure focus. (From Mumenthaler M. Neurological Differential Diagnosis. 2nd ed. Stuttgart, Germany: Georg Thieme; 1992:56, Fig. 20. Reprinted by permission.) (1) Jacksonian march—progression of convulsive movements as the epileptiform activity migrates along the motor homunculus ii. supplementary motor area focus (Brodmann area 6): frequent, brief seizures occurring in clusters with a rapid onset and offset; posturing usually involves the contralateral upper extremity and contraversive head and eye deviation {fencer’s position} iii. premotor cortex focus (Brodmann area 6) and frontal eye field focus (Brodmann area 8): cause unilateral forced gaze and head deviation {version seizures}, which are reliably contralateral only when the focus is in the frontal eye field (1) may involve a decrease in the level of consciousness, which would be better classified as a complex-partial seizure iv. insula cortex focus: typical symptomatic progression involves abnormal laryngeal sensation, followed by dysarthria with perioral and/or contralateral body paresthesias, and finally contralateral convulsions in the face and/or arm (Box 3.3) v. frontal operculum focus: causes bilateral facial movements accompanied by mastication, salivation, and swallowing after an aura consisting of epigastric sensations, fear, and/or autonomic phenomena; may cause speech arrest or vocalizations with foci in either hemisphere, or a Broca’s-type aphasia with a focus in the dominant hemisphere vi. dorsolateral prefrontal cortex focus: causes contralateral forced head and gaze deviation Gelastic Seizures b. simple partial sensory seizures i. parietal lobe focus: most seizure foci are in the primary somatosensory cortex (Brodmann areas 3-1–2), which causes contralateral tingling and/or numbness according to the sensory homunculus that frequently progresses to frank motor seizure activity after a period of general tremulousness (1) sensory symptoms can have a Jacksonian march equivalent (2) negative seizure phenomena with parietal seizures (a) loss of awareness of a body part {asomatognosia} can occur with nondominant hemisphere seizures (b) receptive- or conductive-like aphasias can occur with dominant hemisphere seizures (3) pain or temperature sensations, or bilateral sensations suggest involvement of the secondary somatosensory cortex (Brodmann area 40) (4) the sensation of vertigo (10%), sinking, choking, or nausea indicates inferior parietal lobe involvement (5) changes in affect may also be associated with parietal lobe seizures ii. primary visual cortex focus (Brodmann area 17): causes elementary visual sensations (flashes, scotoma, hemianopia, amaurosis) (1) seizures often involve tonic eye contraversion, oculoclonic, or nystagmoid eye movements, forced eye closure, eyelid fluttering, sensory hallucinations of eye movements, or even the sensation of eye pain iii. occipito-parieto-temporal junction focus: causes complex imagery or hallucinations; frequently visualized objects are recognizable but are distorted in size {micropsia, macropsia} and/or shape {metamorphopsia} iv. temporal lobe focus: two thirds of seizure foci are mesial; one third are lateral (i.e., neocortical)
Seizures and Epilepsy
I. Diagnostic Testing
Box 3.2
 Low-recurrence risk with idiopathic or cryptogenic seizures and a normal EEG (25%)
Low-recurrence risk with idiopathic or cryptogenic seizures and a normal EEG (25%)
 Medium-recurrence risk with an abnormal neurological exam, neuroimaging, or EEG (50%)
Medium-recurrence risk with an abnormal neurological exam, neuroimaging, or EEG (50%)
 High-recurrence risk with an abnormal neurological exam, neuroimaging, and EEG (65%)
High-recurrence risk with an abnormal neurological exam, neuroimaging, and EEG (65%)
 The likelihood of developing epilepsy is increased by having antecedent conditions associated with an increased risk of epilepsy, seizures occurring during sleep, a family history of seizures, an abnormal EEG, particularly one with epileptiform abnormalities, or partial seizures
The likelihood of developing epilepsy is increased by having antecedent conditions associated with an increased risk of epilepsy, seizures occurring during sleep, a family history of seizures, an abnormal EEG, particularly one with epileptiform abnormalities, or partial seizures
II. Partial Seizures
Box 3.3
 Related to hypothalamic hamartomas but EEG identifies epileptiform activity in frontal or temporal lobes
Related to hypothalamic hamartomas but EEG identifies epileptiform activity in frontal or temporal lobes
 Laughter begins in childhood; later involves drop attacks, generalized seizures, cognitive impairment, precocious puberty, and behavioral disorders
Laughter begins in childhood; later involves drop attacks, generalized seizures, cognitive impairment, precocious puberty, and behavioral disorders
 Treatment: lamictal or clonazepam; surgery, particularly stereotaxic
Treatment: lamictal or clonazepam; surgery, particularly stereotaxic
Feature | Complex Partial | Absence |
Aura | Yes | No |
Hyperventilation-inducible | No | Yes |
Photic stimulation-inducible | No | Maybe |
EEG | Temporal spike & wave | Diffuse 3 Hz spike & wave |
Postictal confusion | Yes | No |
Duration | Minutes | Seconds |
Automatisms | Present | Only if prolonged |
(1) causes auditory or olfactory hallucinations, emotional, or psychic symptoms (e.g., flashbacks, déjà vu, jamais vu), sensations of vertigo, and autonomic changes (e.g., epigastric rising sensation, GI upset with nausea and hypermotility, respiratory arrest)
(a) olfactory hallucinations are usually unpleasant, indicating medial temporal lobe involvement {uncinate fits}
(b) gustatory sensations suggest involvement of the temporal opercular cortex
(2) rare manifestations include forced thinking (a rapid recollection of past life experiences), depersonalization, extremes in affect, or rage
2. Complex partial seizures: involves some impairment of consciousness without tonic-clonic activity; does not have to involve a complete loss of consciousness (Table 3–1)
a. general pathophysiology: 40% of cases have seizures that are initiated by a temporal lobe focus, either mesial or neocortical
b. general symptoms: can begin with a simple partial seizure that serves as an aura, or with outright impairment of consciousness
i. complex partial seizures often involve coordinated involuntary movements
(1) reactive automatism: a reaction to the environment (i.e., drinking from a cup that is placed in the hands)
(2) perseverative automatism: the persistence during a seizure of a complex action that was being performed before the seizure onset
c. mesial temporal cortex seizure foci: typically begins with auras consisting of epigastric sensations, fear, and/or oro-alimentary automatisms
i. mesial temporal/hippocampal sclerosis
(1) subtypes: do not correlate with seizure severity or responsiveness to treatment
(a) classic mesial temporal sclerosis: cell loss in the hilum of the dentate gyrus, the tip of the CA4 region, and the CA1-subiculum interface; the CA2 region is relatively preserved (Box 3.4)
(b) minimal mesial temporal sclerosis: cell loss in the hilum of the dentate gyrus
(c) total mesial temporal sclerosis: cell loss in the dentate gyrus and CA1–4 regions
(2) histology: sclerosis implies neuron loss and gliosis in the affected regions
(a) 50% of cases exhibit bilateral sclerosis but usually it is asymmetric
(b) 20% of cases coexist with another pathological process (e.g., cortical dysplasia, tumor)
(3) pathophysiology: unknown cause but often disease onset is preceded by febrile seizures
(a) atrophy of the mesial temporal lobes exists at the time of seizure onset, and can be observed even in neonates
(b) mesial temporal sclerosis exhibits familial segregation, so it likely has a genetic predisposition
(4) specific diagnostic testing: EEG demonstrates spike-and-wave complexes in the inferior temporal leads or low-voltage beta activity at seizure onset that remains local for several seconds before spreading; best observed with subdural/depth electrodes
d. neocortical temporal foci: seizures often begin with auditory hallucinations, vertigo, or visual misperceptions; language disturbance can occur with foci in the dominant hemisphere
i. musicogenic seizures: a type of reflex seizure (Box 3.5) that is triggered by music (e.g., a particular composition, the sound from a certain instrument, or discussing or thinking about music)
Box 3.5
Reflex Seizure Triggers
 Visual patterns (as in 3% of all epilepsies)
Visual patterns (as in 3% of all epilepsies)
 Tactile stimuli: hot water, body postures
Tactile stimuli: hot water, body postures
 Problem-solving
Problem-solving
 Reading, writing, or speaking
Reading, writing, or speaking
 Specific movements G Startle involving any kind of stimulus
Specific movements G Startle involving any kind of stimulus
(1) not the same as a seizure involving the perception of sound or musical notes {musical partial seizure}, wherein the perceived sound tends to be relative unformed (e.g., ringing, whistling)
e. frontal lobe seizure foci: cause complex and bizarre motor automatisms (e.g., eyelid fluttering, repetitive bilateral movements) that occur in clusters, often with minimal post-ictal confusion; frequently are confused with pseudoseizures
i. frontal operculum focus: causes auditory or visceral auras and visceral motor phenomena (salivation, spitting, vomiting)
ii. cingulate cortex or supplementary motor area (Brodmann area 6) focus: cause asymmetric bilateral posturing movements with automatisms; cingulate foci may cause gesturing, simple sexual behavior, or ictal laughter
iii. frontopolar cortex focus: causes forced thinking, aversive head movements, and/or axial clonic jerks
iv. orbitofrontal cortex focus: causes complex motor automatisms and olfactory hallucinations due to involvement of the cortex overlying the septal nuclei that receives the medial olfactory stria; may involve formed vocalizations (e.g., cursing)
v. dorsolateral frontal cortex focus: causes tonic posturing and speech arrest
3. Diagnostic testing: Scalp EEG gives adequate localization in only 50% of cases with temporal foci, and is even worse with frontal lobe foci; a single EEG demonstrates abnormalities in 40% of possible seizure cases, which is increased to 75% with prolonged EEG monitoring
a. ictal EEG patterns of complex partial seizures
i. rhythmic spike and wave activity, which are bilateral in 30% of cases
ii. rhythmic 5–7 Hz activity without spikes
iii. focal attenuation of background activity
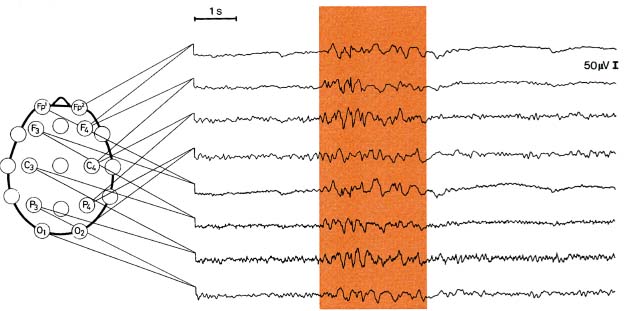
III. Nonsyndromal Generalized Seizures
1. Tonic-clonic seizuresa.
a. symptoms: convulsive activity typically lasts < 1 minute; five phrases include
i. premonition phase: involves mood changes (e.g., irritability) and headaches that may last for days prior to the seizure
ii. immediately pre-tonic-clonic phase: involves a few myoclonic jerks or brief clonic seizure activity; occasionally begins with forced eye and head deviation {aversive movements}
Figure 3–3 Generalized tonic-clonic seizure. (From Mumenthaler M. Neurological Differential Diagnosis. 2nd ed. Stuttgart, Germany: Georg Thieme; 1992, Fig. 1, p. 1. Reprinted by permission.)
iii. tonic phase: contracture of the axial musculature with upward eye deviation, pupillary dilation, and forced expiration of air {epileptic cry}; usually involves some decerebrate posturing
(1) tongue and jaw muscle tonus causes perioral injury, typically lateral tongue biting
(2) frequently patient becomes cyanotic, tachycardic, and hypertensive
iv. clonic phase: starts as low-amplitude, high-frequency (≈ 8 Hz) convulsive movements of the extremities > thorax and abdomen that progresses to high-amplitude, low-frequency (≈ 4 Hz) movements
(1) development of atonia breaks the seizure and causes incontinence
(2) bilaterally asynchronous movements may be caused by separate unilateral seizure foci, but this is rare
v. post-ictal phase: patient is poorly responsive and hypotonic; confusion and memory impairment may last a few minutes to hours, occasionally followed by psychiatric changes (depression, psychosis, anxiety, irritability) that can persist for a day
(1) post-ictal phase involves generalized fatigue, soreness, and migraine headaches
(2) laboratory testing during the post-ictal phase demonstrates respiratory-metabolic acidosis, hyperglycemia, and mild cerebrospinal fluid pleocytosis
b. epidemiology: unlike most other seizure types, occurs with similar incidences across all ages
c. diagnostic testing (Fig. 3–3)
i. ictal EEG: bilateral hemispheric involvement from the seizure onset that is bilaterally synchronous; refined computer analysis may identify asynchronous ictal onset, but this is not necessarily exclusive of a primarily generalized seizure
(1) immediate pre-tonic-clonic phase: any myoclonic or clonic activity is represented as a generalized burst of spike or polyspike activity
(2) tonic phase: identified by an abrupt decrease in voltage with diffuse 20–40 Hz activity {desynchronization pattern}; 8–10 Hz sharp waves build in amplitude during the tonic phase
(3) clonic phase: 8–10 Hz sharp waves of the late tonic phase are replaced by polyspike-and-wave pattern that slows to 4 Hz
(4) post-ictal phase: diffuse suppression of EEG activity with a low-voltage delta frequency predominance
(1) subtypes of interictal abnormalities: often multiple subtypes occur in the same patient
(a) multifocal spike complexes
(b) 3–5 Hz spike-and-wave complexes (usually are bilateral with frontocentral predominance)
(c) irregular spike-and-wave complexes
(2) anticonvulsants tend to slow the background rhythm, except for barbiturates and benzodiazepines, which characteristically increase the beta activity in the 14–20 Hz range
iii. cerebrospinal fluid: any post-ictal pleocytosis should be assumed to be caused by an inflammatory process until proven otherwise
2. Tonic seizuresa.
a. symptoms: diffuse contraction of the axial musculature, sometimes involving the proximal limbs or proximal and distal limbs; paralysis of respiration causes apnea
i. often results in the patient falling {drop attack}, so tonic seizures are frequently confused with atonic seizures
ii. startle seizures
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree