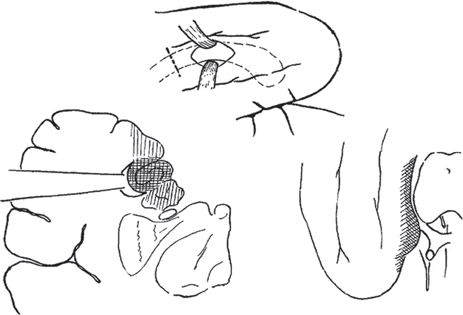
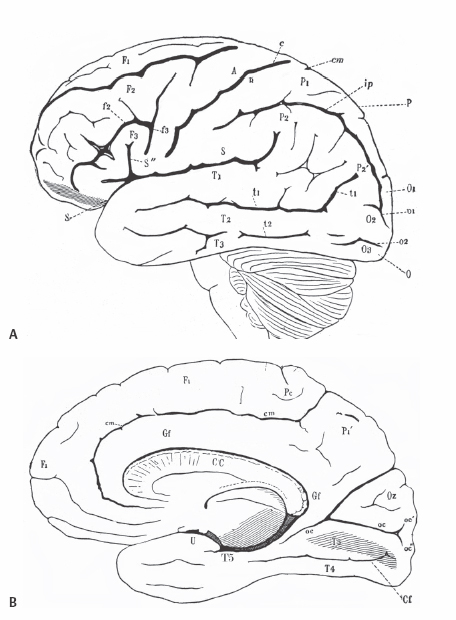
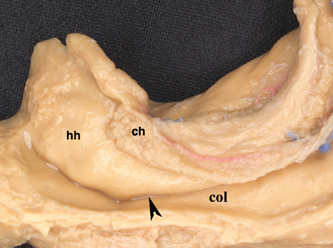
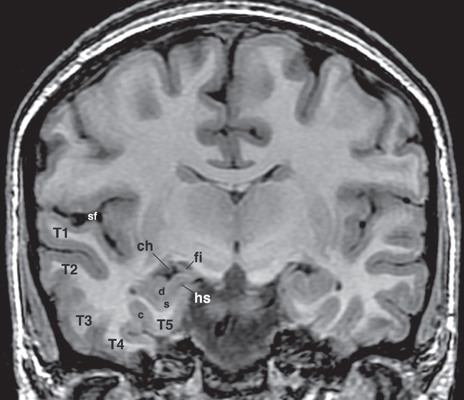
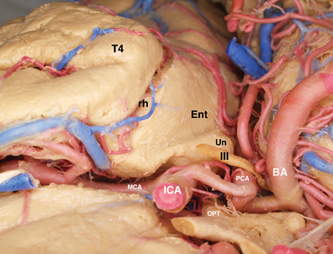
5 Selective Amygdalohippocampectomy It is useful to categorize temporal lobe epilepsy into one of two types based on the anatomical site of seizure onset: neocortical (lateral temporal) or mesial temporal lobe epilepsy (MTLE). Although they share many features that may make diagnosis difficult in a single patient,1, 2 there are sufficient distinguishing characteristics for MTLE with hippocampal sclerosis to be considered a distinct syndromic entity.3 In general, MTLE more commonly displays the typical temporal lobe seizure elements of an aura, staring, automatisms, and posturing.4 MTLE is more related to childhood febrile convulsions, especially prolonged and complicated febrile convulsions.5 The imaging hallmark of MTLE is mesiotemporal sclerosis (MTS).6 Neocortical epilepsy may manifest signs related to perisylvian structures such as a simple auditory hallucination or, in the dominant hemisphere, postictal aphasia.7,8 Although localization of the temporal lobe seizure focus based on a noninvasive evaluation is often complex and may be unreliable,9–11 when history, seizure semiology, electroencephalogram (EEG), and imaging findings point to MTLE, there can be a high degree of diagnostic certainty.10,12 Intracranial electrode monitoring may be required in a subset of patients to confirm the site of seizure onset. Our approach at West Virginia University is to reserve invasive studies for patients in whom the diagnosis of temporal lobe epilepsy or lateralization of the seizure focus is in doubt. In the 1950s, Paolo Niemeyer introduced the concept of selective amygdalohippocampectomy (SAH) (Fig. 5.1).13 His description of a small corridor through T2 to the temporal horn for the selective disconnection of the mesial temporal structures represented a dramatic shift from the temporal lobectomy popular at the time. In fact, as a result of research performed by Scoville and Milner14 and Penfield and Milner15 that stressed the important role of the hippocampus in memory, the trend at the time was to preserve the hippocampus. In the first published report of surgery for temporal lobe epilepsy, Penfield and Flanigin largely preserved the mesial temporal structures in the patients on which they operated.16 Despite concerns of disrupting memory function, even in the early history of surgery for temporal lobe epilepsy, experimental evidence was mounting for the paramount role of the mesial structures in seizure genesis.17–21 Much of this work was presented at the International Colloquium on Temporal Lobe in Marseille in 1954.22 Penfield and Jasper showed that further removal of the hippocampus could convert a failed surgery into a success.23 Removal of mesial temporal structures with the temporal lobectomy was shown to be successful with longterm follow-up by Morris in 1956.24 Feindel and colleagues demonstrated the role of the amygdala in seizure generation and temporal lobe automatisms.25,26 More recently, considerable interest was raised for SAH after Yasargil et al developed an approach using the transsylvian route.27 In Yasargil’s technique, the arachnoid over the sylvian fissure is divided and the bottom of the circular sulcus exposed. An incision between two opercular temporal arteries exposes the ventricular horn allowing the hippocampal formation to be resected by an extrapial approach and the amygdala removed by subpial aspiration. More recently, Hori et al have described a subtemporal approach through the paraphippocampal gyrus.28 Over many years now, a transcortical approach through the second temporal gyrus, similar to that demonstrated by Niemeyer, has been used at the Montreal Neurological Institute and now at West Virginia University.29 The SAH approach is based on the concepts first developed from Hughlings Jackson’s description of a lesion in the uncus causing psychomotor seizures (Jackson called this a “dreamy state”) and the role played by the mesial temporal structures in human epilepsy.30 Subsequently, an abundance of experimental studies have pointed to a very important role of the mesial temporal structures in experimental and human epilepsies.17,18,20,23,24,31,32 Results from many patients operated for MTLE with SAH confirms the overwhelming participation of the mesial temporal and limbic structures in temporal lobe epilepsy.33–35 Furthermore, recently the International League Against Epilepsy determined that MTLE represents a sufficient cluster of signs and symptoms to comprise a specific epilepsy syndrome.36 The goal of SAH is to achieve the best possible results on the seizure tendency in the most selective manner, and to spare from resection those cerebral structures not involved in the seizure focus. At centers with experience in SAH, the results on seizure tendency clearly rival the results of corticoamygdalohippocampectomy (CAH) (see below).27–29,32,35 Although the neuropsychological advantages of SAH have yet to be entirely proven,37–39 the intuitive advantages are inescapable. Sparing brain tissue not involved with the seizure focus should be an important goal of epilepsy surgery. Fig. 5.1 Niemeyer’s original description (1957) of the SAH.13 A trans-T2, transventricular approach for selective removal of the mesial temporal structures. It is essentially the identical approach used today. Nineteenth-century neuroanatomists developed a numbering system for the gyri and sulci of the brain that today is still the preferred method of nomenclature.40,41 It is a simple but useful system that emphasizes the concept of gyral ribbons interconnecting the convolutions of the brain.41–43 There are five gyri running the length of the temporal lobe. The superior temporal gyrus is T1, middle temporal gyrus T2, inferior temporal gyrus T3, fusiform gyrus T4, parahippocampal gyrus T5, and the most medially located gyrus in the temporal lobe is the hippocampal complex (composed of the hippocampus proper and dentate gyrus) (Fig. 5.2A). Similarly, the temporal gyri are separated by four longitudinal sulci, which are: S1, S2, S3, and S4. The superior temporal gyrus (T1) is bounded above by the sylvian fissure. Below T1 the superior temporal sulcus (S1) is the deepest sulcus in the temporal lobe extending toward the temporal horn, and it is an important anatomical guide to localizing the ventricle. T4 is well demarcated from the parahippocampus (T5) by the constant and strong collateral fissure (S4) that produces an obvious bulge into the ventricle of the temporal horn called the collateral eminence (Figs. 5.2B, 5.3, 5.4). Fig. 5.2 (A) Brain convexity showing the numbered system of nomenclature. T1, T2, and T3 are visible on the lateral surface. (B) On the medial brain surface, T4, T5 (parahippocampus), and uncus (U) are plainly visible. The fifth temporal gyrus is better known as the parahippocampal gyrus because of its close relation to the hippocampus proper (Fig. 5.2B). The parahippocampus lies adjacent and inferolateral to the hippocampus. The parahippocampus is clearly delineated laterally by the collateral fissure (S4) and mesially by the hippocampal sulcus. Its anterior extent does not reach the temporal pole but ends ~2 cm behind it. Peter Gloor defines the entorhinal cortex in man to be the region of parahippocampus anterior to the most posterior limit of the uncus.44 Posteriorly, the parahippocampal gyrus is divided into two branches by the anterior calcarine sulcus. The superior branch merges into the isthmus of the cingulate gyrus, and the inferior one becomes the lingual gyrus of the occipital lobe. Fig. 5.3 Dissection of the left temporal lobe. The view is into the opened temporal horn. The arrowhead points to the lateral ventricular sulcus that is the entry into the parahippocampus. ch, choroid plexus; col, collateral emminance; hh, hippocampus head. The uncus has a conical shape (Fig. 5.5). Its medial convex surface can be further subdivided into several small gyri including the semilunar gyrus superiorly, ambient gyrus, uncinate gyrus, band of Giacomini, and intralimbic gyrus that form its posterior apex. In fact, the rostral end of the hippocampus and dentate bends medialward to form most of the uncus. The anterosuperior uncus (semilunar gyrus) is occupied by the amygdala. The hippocampus proper envelops the hippocampal sulcus (Fig. 5.4). Its two pial walls closely adhere to each other and contain the vascular supply of the hippocampus. The superomesial wall of the sulcus is formed by the dentate gyrus, whereas the subiculum and hippocampus proper form the inferolateral wall (Fig. 5.4). With the ventricle opened, the hippocampus appears as a distinct bulge on its inner surface covered by a white matter tract, the alveus, and a glistening ependymal layer (Fig. 5.3). The hippocampus lies just lateral to the cerebral peduncle. Its anterior portion, or head, is short and occupies the rostral extent of the temporal horn and curves mesially to form the bulk of the uncus. Its middle part (body) extends posteriorly into a narrower part called the “tail” that curves backward and upward to the trigone of the ventricle. The fimbria is a white matter band that runs horizontally along the medial border of the hippocampus, just above the dentate gyrus, separated by the fimbriodentate sulcus. Its free aspect forms the inferolateral edge of the choroidal fissure. It ends anteriorly at the posterior gyrus of the uncus, the intralimbic gyrus (Fig. 5.4). Posteriorly, under the splenium, the fimbria becomes the fornix. Fig. 5.4 Coronal T1 image at the level of the hippocampal body. T1 to T5 label the temporal gyri. c, collateral sulcus; ch, choroids plexus; d, dentate gyrus; fi, fimbria; hs, hippocampal sulcus; s, subiculum; sf, sylvian fissure. The choroid plexus partially covers the hippocampus as they both bulge into the temporal horn and is an important surgical landmark for orientation while working in the ventricle (Fig. 5.3). The choroid plexus arises from the tela choroidea, an ependymal and pial layer that fill the choroidal fissure. The tela choroidea spans the space between the fimbria and the stria terminalis, attached to the two white matter bundles (Fig. 5.4). The anterior choroidal artery enters the temporal horn at the inferior choroidal point to form the choroid plexus. Therefore, the choroid plexus can be reflected posteriorly to see the intralimbic gyrus. Tilting the choroid plexus laterally exposes the stria terminalis, and mesial retraction uncovers the fimbria and hippocampus. Fig. 5.5 Dissection of the left medial temporal lobe, ambient cistern, and related structures. BA, basilar artery; Ent, entorhinal cortex (anterior extension of parahippocampus); ICA, internal carotid artery; III, cranial nerve III; MCA, middle cerebral artery (M1 branch); OPT, optic tract; PCA, posterior cerebral artery; rh, rhinal sulcus; T4, fusiform gyrus; Un, uncus. The mesial temporal structures are defined as including the uncus, amygdala, hippocampal complex, fimbria, parahippocampus, and entorhinal cortex. The collateral sulcus and choroid plexus are important landmarks that define the mesial temporal area. The anatomical region of the mesial temporal area lies between the choroid plexus and collateral sulcus, also including the more anterior structures of the amygdala and uncus. Therefore, the lateral temporal region (often called iso- or neocortex to contrast with the archicortex named for the phylogenetically more primitive threecortical-layered hippocampus) comprises the temporal lobe lateral to the collateral fissure. The parahippocampus (and especially the subiculum) is the transition between the six-layered neocortex and three-layered archicortex of the hippocampus. Temporal lobectomy consists of a removal of the entire temporal lobe including the mesial structures. This complete removal of the temporal lobe is rare today. Therefore, to identify the typical anterior, lateral, and mesial structure resection, it is preferable to use the terms anterior temporal resection or corticoamygdalohippocampectomy to more accurately describe the extent of structures that have been removed. This is the standard temporal resection performed at most epilepsy centers. It essentially corresponds to an anterior temporal neocortical resection followed by a removal of the temporal mesial structures. Variations on this approach have been described including anatomically standardized resections45,46 as well as a tailored type of operation.47 Our approach to CAH is strictly image guided to localize anatomical landmarks that define the resection margins. Essentially, in the dominant hemisphere along T1 the resection extends no further posterior than the precentral sulcus (corresponding to ~3.5 to 4 cm), and in the nondominant hemisphere the posterior extent of the T1 removal is the central sulcus (corresponding to ~4 to 4.5 cm). The lateral cortex is removed en bloc as a specimen. The temporal horn is opened, and the mesial structures are disconnected exactly as is described below for the SAH. As the name implies, CA consists of an anterior temporal cortical resection combined with an amygdalectomy, emptying of the uncus, and sparing of the hippocampal formation. The neocortical resection is limited as described for CAH. In the CA, the cortical resection is carried up to the collateral sulcus. The anterior temporal horn is opened for the purpose of identifying landmarks. The hippocampus and the adjacent parahippocampal gyrus are left undisturbed. The amygdala is resected as completely as possible, and the uncus is emptied in a subpial fashion. However, it is unavoidable to encroach on the anterior most part of the hippocampus that reflects back upon itself within the uncus. This surgical approach is used mainly in patients who have failed the ipsilateral sodium amytal test for memory and/or are at risk for a significant functional memory decline from a disconnection of the mesial temporal structures. CA has been shown to be useful for controlling the seizure tendency when the hippocampus retains significant memory function and cannot be included in the resection.48,49 The SAH was introduced by Niemeyer to treat temporal lobe epilepsy arising from the mesial temporal structures (Fig. 5.1).13 Subsequently, the overwhelming importance of MTLE in medically intractable temporal lobe epilepsy has been established. Building on the work of earlier investigators, modern reports of SAH have confirmed its utility for the treatment of mesial temporal structures.29,32–34 To date, SAH has become the standard approach to treat pharmaco-resistant MTLE at many epilepsy centers, including ours. The scalp and bone exposure for SAH can range from a frontotemporal craniotomy to a keyhole approach. A larger craniotomy is required for electrocorticography (ECoG), and a keyhole approach is preferred if ECoG is not performed. All aspects of the SAH are enhanced with the use of image guidance, and in keyhole surgery image guidance is required. The patient’s head is immobilized in the three-pronged headholder. Anesthesia is general endotracheal. A roll is placed under the ipsilateral shoulder with the head turned toward the opposite shoulder. The vertex being lowered inferiorly allows the surgeon better access to the mesial structures, and when turned ~30 degrees from horizontal improves visualization of the structures more posterior in the ventricle. The traditional frontotemporal craniotomy is exposed via a curvilinear question-mark–shaped scalp incision that starts at the zygoma, extends posterior just to the back of the ear, up to the superior temporal line, and anterior to the hair line. The scalp is reflected anterior with the temporalis muscle and retracted with hooks. The root of the zygoma must be visualized to confirm an adequate exposure to the floor of the middle fossa. A frontotemporal bone flap is elevated plus a craniectomy of the temporal bone is performed with a rongeur to the zygoma inferiorly and anterior to the apex of the middle fossa, if possible. An adequate bone opening is essential to allow a sufficient exposure of the temporal lobe and to fit the 16-channel ECoG, if required. The dura is reflected away from the temporal lobe. Once the brain is exposed, important sulcal and gyral landmarks are identified, namely the sylvian fissure, T1, T2, and the central sulcus. A corticectomy measuring 2 to 3 cm is made along the upper border of the second temporal gyrus (T2) and just below the superior temporal sulcus (S1) (Fig. 5.6). The posterior extent of the corticectomy is the central sulcus on the nondominant side and the precentral sulcus in the dominant hemisphere. Image guidance is required to localize these sulcal landmarks that correspond to ~3.5 cm in the dominant hemisphere and 4.5 cm in the nondominant hemisphere from the temporal tip. If necessary, a small gyrectomy of T2 can be performed to facilitate maneuvers within the ventricle. Using an ultrasonic dissector at very low settings of suction and vibration, a corridor of ~3 to 4 cm in height is fashioned down to the ependymal lining that is opened (Fig. 5.6). The corridor is fashioned along and directed by the inferior wall of S1 that points to the temporal horn. A retractor is then inserted to provide an unobstructed view of the temporal horn and of the mesial temporal structures (Fig. 5.7). The ventricle is open completely from its anterior tip to the posterior limit of the corridor exposure. Three ependymal-lined structures are now seen bulging into the ventricle. On the lateral surface is the collateral eminence and below is the hippocampus. Between them is the lateral ventricular sulcus (Fig. 5.3). Anterior to the choroid plexus and arising from the medial wall of the ventricle is the amygdala. The choroid plexus is an important intraventricular landmark that should be identified early. Often the hippocampus must be retracted laterally to see the choroid plexus, which is followed to its anterior origin in the choroidal fissure (inferior choroidal point) that marks the posterior limit of the uncus. The entire mesial resection is endopial/subpial using the ultrasonic aspirator at its lowest settings of suction and vibration (using the CUSA (Integra Life Sciences, Plainsboro, NJ), suction and vibration are set at 10). The resection proper is begun by entering the lateral ventricular sulcus at the junction of the hippocampus with the collateral eminence (Fig. 5.3). This is the entry into the parahippocampal gyrus that is emptied subpially between the hippocampal sulcus and the collateral sulcus. This maneuver separates the hippocampus from its lateral connection and allows the hippocampus to be retracted laterally to facilitate subpial aspiration of the fimbria and disconnection mesially. The hippocampus is separated from the hippocampal tail posteriorly. Lifting the hippocampus off and separating it from its vascular pedicle, the hippocampal sulcus, completes the en bloc removal. The hippocampal vessels typically can be teased and separated from the hippocampus without coagulation and division. Additional hippocampus can be removed piecemeal posteriorly. The habitual posterior limit of the hippocampal formation resection corresponds to the midbrain tectum. These landmarks and the extent of hippocampal resection are readily identified with image guidance. The anterior extent of the resection corresponds to the level of the rhinal sulcus that includes the entire uncus (Fig. 5.5). The uncus, normally herniated over the edge of the tentorium, should be completely emptied subpial. The third cranial nerve and posterior cerebral artery (P1) are expected to be seen in the cistern beneath the partially transparent uncal pia. The amygdala is removed piecemeal. Essentially, the structures found between the choroid plexus and collateral sulcus are removed entirely, taking care to keep the pial surface intact (Figs. 5.6 and 5.7). The posterior limit is the level of the midbrain tectum identified with image guidance (Fig. 5.8). Anterior to the choroid plexus the uncus is emptied completely. The mesial boundary of the amygdala resection is not precise. Essentially, medial to the level of the choroid plexus, the resection is only subpial aspiration of cortex. Many of the pitfalls in SAH can be avoided by using image guidance, and image guidance provides useful corroboration for landmark identification. The extent of anterior temporal craniectomy and temporal lobe exposure is easily confirmed with image guidance. In the asleep patient, image guidance is required to identify the central and precentral sulci that mark the posterior limit of the T2 corticectomy. On occasion, the dominant draining vein is more related to S1 and does not take the expected course along the sylvian fissure, which must be recognized. Fashioning the corridor to the temporal horn is benefited by image guidance to help define and improve the trajectory to the target. Image guidance enhances the performance of SAH by confirming landmarks, aiding the fashioning of the corridor to the ventricle, and documenting the posterior limit of the hippocampal resection. Image guidance is utilized by the author in essentially all surgeries for epilepsy.
Surgical Anatomy of the Temporal Lobe with Emphasis on the Mesial Structures
Surgery for Temporal Lobe Epilepsy
Temporal Lobectomy
Corticoamygdalohippocampectomy (CAH)
Corticoamygdalectomy (CA)
Selective Amygdalohippocampectomy (SAH)
Surgical Technique of SAH
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








