2. Impaired single-word comprehension
1. Impaired object knowledge, particularly for low-frequency or low-familiarity items
2. Surface dyslexia or dysgraphia
3. Spared repetition
4. Spared speech production (grammar and motor speech)
2. Imaging must show one or more of the following results:
a. Predominant anterior temporal lobe atrophy
b. Predominant anterior temporal hypoperfusion or hypometabolism on SPECT or PET
2. Histopathologic evidence of a specific neurodegenerative pathology (e.g., FTLD-tau, FTLD-TDP, AD)
3. Presence of a known pathogenic mutation
Abbreviations: AD = Alzheimer’s disease; FTLD = frontotemporal lobar degeneration; PPA = primary progressive aphasia.
Other major developments since the mid-1990s have been (a) refinement of the cognitive profile of SD, particularly the impact on reading/writing, object recognition/use, episodic memory, working memory, and perceptual abilities; (b) clearer specification of the distribution of changes on magnetic resonance imaging (MRI); and (c) more information regarding the pathological basis of the condition. In addition there is increasing realization that most, if not all, patients exhibit behavioral changes, which may be subtle at presentation but often have major consequences for caregivers as the disease progresses.
Clinical features
Conversational speech in SD may appear normal in early stages, particularly when patients are talking about familiar topics, because (a) the sound-based components of speech (phonology and prosody) as well as syntax are reasonably well preserved, and (b) the vocabulary associated with such well-known topics is relatively resistant to degradation. As the disease progresses, however, specific and lower-frequency words such as “kettle” or “supermarket” are replaced by more general and higher-frequency terms like “thing” and “place.” Even at early stages, the receptive vocabulary deficit can often be revealed by simple questions such as “How’s your comprehension?” or “Do you have any hobbies?” The standard replies to these questions, even from patients who have been chatting away a moment before, are “Comprehension, what’s that?” and “Hobbies, what are they?” Note the patients’ correct understanding and production of number-marking (“is” for the singular noun comprehension, “are” for the plural hobbies).
Anomia on testing of naming is always present and equally apparent from any modality of stimulus: real object, coloured picture, line drawing, definition, sound, touch, etc. Although naming is usually impaired in the other forms of PPA too, the pattern of anomia in SD is very distinctive. First, the only objects which the patients reliably succeed in naming are highly familiar exemplars that are also typical of their semantic category, such as cat, dog, and horse for animals (see [20] for results from a comprehensive study of naming in SD). For less common items (e.g., crocodile or zebra), the patients substitute either a more familiar exemplar from the same category (e.g., “horse” for zebra) or a superordinate label (“animal”). From moderate stages onwards, the most common naming response becomes “I don’t know” unaccompanied by any identifying information. Asked to name a picture of, or define the word, “caterpillar,” a patient with PNFA may fail on both responses requiring language output, but will often demonstrate with a finger how the caterpillar moves, thereby revealing knowledge of the object concept. This is rarely, if ever, observed in SD.
Anomia in SD is accompanied by the second essential feature, impaired word comprehension. One useful clinical test is to ask the patient first to repeat a long, unusual word such as “stethoscope” or “chrysanthemum” and then to define it. Repetition in SD is almost always correct and rapid, but the definition will be generalized, lacking in detail and, as the disease progresses, the most common response is once again “I don’t know.” In a recent study of 47 consecutive PPA patients, impaired word comprehension – as judged by semi-structured clinical interview and testing using an array of model animals/tools – was found to be the best discriminator of SD as other features, such as severe anomia, can be a feature of lvPPA cases [3].
Another almost universal feature of SD is the presence of surface dyslexia, which refers to the inaccuracy in reading aloud words with an irregular or unpredictable spelling-to-sound correspondence. Atypical or irregular words are “regularized”: for example, the written word sew is read aloud as if it rhymed with all the other English words with the spelling ew (new, few, grew, dew, stew etc.) [21].
Speech in SD is well articulated with normal prosody and an absence of phonological errors. Frank agrammatism is absent, although the lack of content words can lead to patients to produce syntactically odd phrases [22]. For example, an SD patient trying to describe his problem to JRH said, “I want to say you right now that this is what’s wrong.” We infer that he was initially aiming for the verb “tell,” and then failed not only to find the desired word but also to adapt the syntax for the retrieved word, which requires “say to you.”
As a disorder of conceptual knowledge, SD also affects object use, although this is often subtle in the beginning, and carers typically report that the patients function normally with everyday objects at home.
Patients with the rarer variant of SD involving the right temporal lobe more than left have a severe deficit in recognition of people even early in the disease and, at late stages, even personally relevant people may fail to be recognized. This form of prosopagnosia is multimodal, affecting recognition of names and voices as well as faces [15, 18, 23, 24].
In addition to the cognitive symptoms, there are typically behavioral and personality changes. Lack of empathy and mental inflexibility are commonly reported by caregivers. Degraded social functioning results from a combination of emotional withdrawal, rigidity, disinhibition, apathy and/or irritability, as well as the obvious difficulty in dealing with certain aspects of social events that might depend on understanding the things people say and do. Stereotyped activities sometimes develop and may verge on obsessions; clockwatching and an intense interest in jigsaw puzzles or Sudoku are fairly common [25, 26]. Changes in eating behavior are frequent, and often reveal themselves in the development or exacerbation of a sweet tooth [27]. Usually there is a restriction of food preferences, or bizarre food choices, rather than the overeating often seen in bvFTD. Changes in personality and behaviour are more prominent in patients in whom the atrophy pattern is dominated by right-temporal lobe abnormality [18, 28–30].
All of our description so far has, quite naturally, emphasized the impairments; however, an understanding of the nature of any disorder also requires specification of what is unimpaired. Patients with SD have good orientation and recall of recent life events, together with preserved visuospatial and topographical abilities [31, 32]. They do not get lost. Until the disease is moderate/severe, they are often able to engage in complex hobbies such as playing golf or cards.
Neuropsychological findings
In the majority of cases, establishing a diagnosis of SD is straightforward if the clinician observes a combination of severe naming impairment and deficits in word comprehension, in the setting of otherwise generally preserved cognitive abilities. On the Addenbrooke’s Cognitive Examination (ACE-R or ACE-III), for instance, patients show a characteristic profile: very poor naming and fluency (especially for a semantic category but also for words beginning with a particular letter); impaired word comprehension; poor recall of the name and address; preserved orientation and visuospatial skills [31, 33]. The Cambridge semantic memory battery involves a single set of items that are used to assess the status of conceptual knowledge via different modalities of input and output. The tasks in the battery include category fluency, picture naming, naming in response to verbal descriptions, word–picture matching, picture and word sorting, and a probed semantic attribute questionnaire. The battery has proven useful in the evaluation of patients with all forms of FTD as well as AD [34–39]. An updated version of the battery developed for use in patients with PPA (the SYDBAT) involves naming, word-picture matching with multiple semantic distractors, a picture–picture matching task, and word repetition. Patients with SD show a striking and distinctive profile with marked anomia, impairment on the two comprehension tasks but preserved word repetition [40].
On targeted neuropsychological tests, the semantic deficit is most apparent on tasks involving either verbal output (e.g., object/picture naming, category fluency, the generation of verbal definitions to words or pictures) or verbal comprehension (e.g., matching a spoken word to its picture referent). A useful summary is that, whichever of these tests is employed, the level of success in SD is characterized by four variables. The first is the stage of disease. The second is the familiarity of the stimulus, with invariable advantages for objects/concepts higher in this regard. Third is the typicality of the stimulus in its semantic class. Note that typicality is only partially correlated with familiarity: the tortoise and the hare of legendary fame are both lower-familiarity animals, but the hare – which is the more prototypical animal – wins the race in SD [20]. Finally, the specificity of semantic knowledge required by the task also has a profound impact on the patients’ performance. For example, in a word–picture matching task (one spoken word, seven picture alternatives), SD patients may succeed in pointing to the correct picture even for a relatively low-familiarity and low-typicality object like a kingfisher if the incorrect pictures alternatives are things like cigarettes or shoes. Performance falls dramatically, however, when all six foil pictures are different small birds, because this discrimination requires much more specific conceptual knowledge [41].
Non-verbal semantic knowledge is less easy to assess but, if the tests are appropriately designed, the results reveal the same predictable pattern of performance modulated by familiarity, typicality, and specificity. Some non-verbal tests that we and others have designed include (1) matching of object pictures to their characteristic sounds [42, 43]; (2) matching of manufactured artifacts (like a vegetable peeler or a hammer) to their typical recipients or to other objects that could be used for the same purpose [42]; (3) coloring-in of line drawings of objects with characteristic colors [44]; (4) selecting the correctly colored animal/object from two alternatives, such as an orange vs. a green carrot [44]; (5) selecting the correct version of a pictured object or animal from two alternatives when one has been altered in some way (e.g., an elephant with large vs. small ears [45]); (6) delayed copying of line drawings [9, 46–48], and (7) choosing between familiar and unfamiliar tunes [43] .
Figure 12.1 shows examples of drawings by SD patients [48] in two different conditions: (a) when the model drawing remains in front of the patients and they are asked to copy it; (b) when the drawing is shown to the patients for a good long look, then removed and – a mere ~10 seconds later – they are asked “please draw what you were just looking at.” The copies with picture model present demonstrate that SD patients have unimpaired ability to perceive and reproduce a visual stimulus. Performance in the delayed copy task can be interpreted as follows. Even people with healthy brains cannot hold on to a detailed, literal image of a stimulus for 10 seconds; the delayed copy is therefore based not only on short-term visual memory of the stimulus picture but also on semantic knowledge about it. Studying a picture of a camel automatically produces the categorization “camel,” and we know that camels have humps. SD patients do not know that the picture is a camel, but only that it is some sort of animal; typical animals do not have humps, and therefore neither do the patients’ delayed copy drawings. No birds have four legs but typical animals do, and this generalized, typical information is again reflected in the patients’ delayed-copy drawings of a duck.
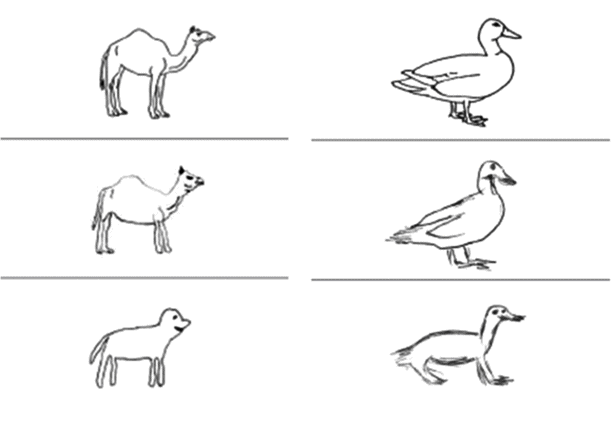
Two examples of direct copy (middle row) and delayed copy (bottom row) of stimulus line drawings (top row) of a camel – showing loss of correct but atypical detail and evolution towards a generic four-legged animal; and a duck – showing the addition of an incorrect feature that is typical of many animals.
As demonstrated by Patterson et al., [47] the same factors of familiarity and typicality modulate levels of success even in apparently non-semantic tasks like reading words aloud, writing them to dictation, and turning stem (present-tense) forms of verbs into their past-tense forms.
Studies of SD have contributed significantly to our understanding of the cognitive and neural architecture of semantic memory. We argue that, within the widespread semantic network in the brain, the anterior temporal lobes represent a component that coordinates and links information from all modalities of input and to all modalities of output. Deterioration of this “hub” will, therefore, have similar consequences for both objects and words (for fuller discussion see [9, 47, 49, 50]). This view is not universally accepted and other theorists maintain that object and word knowledge are represented in separate brain systems/regions, and thus conclude that patients with SD have two separate deficits [51, 52].
Investigations of object usage in SD are pertinent to this debate. Some theories propose that there is a separate “action semantic” system, which can be spared when there is insufficient knowledge to support naming and even non-verbal kinds of responding like sorting, word–picture matching or associative matching of pictures or words (e.g., [53, 54]). This view is promoted by anecdotal reports that patients with SD who fail a whole range of laboratory-based tasks of the latter kind still function fairly normally in everyday life. There are two reasons for this apparent discrepancy. First, the objects used appropriately at home are mostly common ones, like forks and combs and socks; as already emphasized, familiarity is a pervasive and powerful influence in every aspect of SD. In fact, these common objects may also engender correct responses on more formal tests. Less common objects, such as a stethoscope or a corkscrew, that are nonetheless easy for controls or other sorts of patients will reveal the deficit of object recognition/use in SD [55–57]. Secondly, patients with SD do significantly better with their own exemplars of objects in their familiar home settings. Snowden et al. [58] provided the first dramatic report of this phenomenon, confirmed later by other studies [57]. SD patients asked to name or demonstrate the use of their own kettles in their own kitchens had much greater success than (a) when confronted with a different but equally ordinary exemplar of a kettle in the laboratory/clinic, and (b) even when confronting their very own kettles in unfamiliar locations. Later studies demonstrated both a striking degree of impairment in the use of less familiar objects and a strong concordance between the patients’ ability to use a specific object and their conceptual knowledge of it as indexed by performance on the other semantic tasks [56, 59].
Other forms of memory: episodic, autobiographical, and working memory
Performance on tests of verbal anterograde memory, such as logical memory (story recall) and word-list learning, is uniformly poor and is largely secondary to the patients’ poor semantic knowledge of the words to be encoded. By contrast, patients with SD often score within the normal range on non-verbal memory tests such as recall of the Rey Complex Figure [36]. In study–test designs, they also show good recognition memory (i.e., in response to “Did you see this before?” or “Which of these did you see before?”) for photographs of objects or famous faces [60, 61]. Intriguingly, however, the patients’ success in these tasks relies heavily upon perceptual information. Independent of whether the objects or people represented in the stimulus pictures are ones that the patients still know fairly well (as indexed by other tests of their knowledge of these items), the patients achieve normal or near-normal recognition memory as long as the pictures shown at study and test are identical. If the test items represent the same objects or people but photographed from different angles or presented in different colours or (especially) if objects are different exemplars of the same thing, SD patients are significantly impaired in recognition memory for items of which they have degraded conceptual knowledge [60, 62]. These studies concluded that patients with SD are unusually reliant upon perceptual inputs to medial temporal episodic memory structures, whereas normal subjects can use both semantic and perceptual features to encode new information.
Despite the relatively preserved recognition memory for perceptually identical material in SD, it could be argued that this is not a true reflection of episodic memory as conceptualized by Tulving [63, 64] who emphasised the “what, where and when” elements fundamental to memory. In an attempt to address this issue, we designed a more naturalistic task with incidental encoding of information [65]. A researcher visited patients in their homes on two consecutive days. On day 1, the patients were given a number of standard neuropsychological tasks but with the covert intent of exposing the patients to information that could form the basis for tests of episodic memory the following day. On day two, the patients were unexpectedly asked to remember various aspects of the experience, including spatial (e.g., “Did I sit in this chair?”; “Did we do this test in the kitchen or the living room?”), temporal (e.g., “Did we do this test first or second?”), and perceptual (e.g., “Was I wearing a brooch yesterday?”). SD patients, even those with quite advanced disease, remembered most of this information at a level equivalent to controls. This study supports Elizabeth Warrington’s early observations on the preserved status of episodic memory in SD.
Investigations of autobiographical memory in SD have yielded somewhat mixed results. The first empirical study documented a reverse temporal gradient or, more accurately, a step function whereby recent memories are relatively preserved in comparison with impoverished remote memories [66]. This pattern is opposite to the temporal gradient observed in Alzheimer’s disease, where recent memory is most impaired. Subsequent work has confirmed this basic profile in SD: although memory for recent events has not always been unimpaired (see for example [67, 68]), it is usually superior to memory for more distant personal life events [69–73].
Digit span, both forwards and backwards, is almost invariably within the normal range and indeed sometimes at the upper end of this range: one of the first patients with SD in Cambridge whom we studied extensively and longitudinally, at an advanced stage of decline where she could not name a single object, had a forward digit span of 9 and a backward digit span of 6 [74]. This result demonstrates intact mechanisms of phonological working memory in SD, but also arises from the fact that simple number knowledge is well preserved in this condition [75, 76], as becomes apparent when the patients are given memory span tasks not with digits but with ordinary words. If the stimulus sets are composed either of words whose meanings are still relatively well known to an individual patient or of words with degraded meanings for that same patient, the outcome is dramatic. Immediate serial recall of 3, 4 or 5 “known” words is good; the same task with familiar but, for that patient, now “unknown” words is very impaired, with frequent transposition errors, e.g., the two consecutive words “mint, rug” reproduced as “rint, mug” [77–80]. This phenomenon has been characterized as the loss of semantic “binding” of phonology.
Imagining the future
The conception of episodic memory has undergone a revision over the past 5 years, reframing it from a predominantly past-oriented system to one that appears equally important for future-oriented thought [81]. A number of studies have reported marked impairments of this ability in SD, despite relatively good retrieval of at least recent past memories. This asymmetric impairment of future with respect to past oriented thinking in SD has been found when patients attempt to form self-representations [82] and to construct possible personally relevant events at a future time point [83]. Notably, the events generated by SD patients tend to represent “recast,” or previously experienced, scenarios from the past, suggesting that semantic memory may be crucial for the construction of novel events [84]. In Alzheimer’s disease (AD), by contrast, damage to the episodic memory system produces equivalent impairments in autobiographical retrieval and simulation of personal future events [83, 85]. The deficit in future thinking in SD correlates robustly with the degree of atrophy of the left anterior temporal lobe [83] suggesting that semantic representations may function as “scaffolding” which provides structure and meaning to future simulations [83, 86].
Social cognition and emotion
Systematic investigations of emotion processing in SD are recent but show severe emotion recognition deficits, irrespective of the modality of testing [87]. Patients are poor at recognizing emotions from faces, words or music, with negative emotions more severely affected than positive ones [88–91]. Importantly, this reflects a primary emotion-processing impairment that is not attributable to other cognitive deficits [92, 93]. Brain imaging analyses indicate that deficits in emotion recognition are due to atrophy in emotion-specific brain regions, distributed across the frontal and temporal lobes [89, 91, 94]. At a practical level, these difficulties in understanding emotions may lead to a lack of intimacy between spouses, evidenced by abnormal levels of mutual gaze between semantic dementia patients and their spouses [95]. Impairments in emotion processing also result in significantly increased levels of carer burden [96].
Most theory-of-mind (ToM) tasks, including the social “faux pas,” reading-the-mind-in-the-eyes, and the first/second order belief tests, are difficult to administer in SD because the materials require comprehension of quite complex vocabulary. Two studies have attempted to circumvent these problems by using visually based tests. Eslinger et al. [97] reported that a mixed sample of SD and progressive non-fluent aphasic cases showed significant deficits in identifying the thoughts and feelings of cartoon characters in social situations. More recently, Duval et al. [98] demonstrated marked impairments across cognitive and affective measures of ToM in a sample of predominantly left-lateralized SD patients. We have confirmed this finding but showed a strong association with atrophy of the right anterior temporal lobe [99].
Structural and functional imaging
The most striking and consistent neuroanatomical finding in SD is focal, often severe, often asymmetric (typically left more than right) atrophy of the anterior and ventral portion of the temporal lobe. At a clinical level this is easiest to spot on T1 coronal images and can be missed in the early stages if such images are not obtained. Typical examples are shown in Figure 12.2.
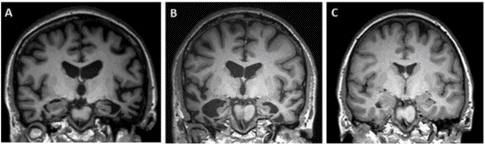
Illustrative T1-weighted coronal MRIs at midhippocampus showing A. an SD patient with predominantly left-sided temporal atrophy: note severe involvement of the fusiform gyrus and enlargement of the temporal horn of the lateral ventricle; B. an SD patient with similar but predominantly right-sided temporal atrophy ; C. a healthy age-matched control.
Early studies, based upon visual inspection, suggested involvement of the polar and inferolateral regions with relative sparing of the superior temporal gyrus and of the hippocampal formation [15]. Subsequent studies using methods of quantification (both voxel-based morphometry and manual volumetry of defined anatomical structures) have clarified a number of issues. First, although defects may appear to be strikingly unilateral, volumetric assessment establishes that, even if asymmetric, atrophy is bilateral in all cases, even early in the course of the disease [100–102]. Second, the regions most profoundly affected are the temporopolar and perirhinal cortices, which occupy the anterior fusiform gyrus [101]. Third, extent of semantic impairment correlates with the degree of anterior temporal atrophy – and hypometabolism in the anterior temporal lobe [19, 101, 103–107], especially in the anterior portion of the fusiform gyrus, subjacent to the head and body of the hippocampus [19].
The status of the hippocampus and functionally related parahippocampal structures (notably the entorhinal cortex) has been a topic of debate, particularly given relatively good episodic memory in SD. Despite early suggestions of relative sparing of the hippocampus [108], volumetric analyses have shown asymmetric atrophy of the hippocampus, which, on the side of predominant neocortical atrophy, is typically as severe if not more so in SD than in AD when patients are matched for disease duration [101, 106]. In AD, the loss of volume tends to be symmetrical in terms of both left–right and rostral–caudal distribution. In SD, there is both lateralized asymmetry and front–back asymmetry (virtually always rostral more severe than caudal) [100, 104, 106].
Recent developments in MRI technology have extended our knowledge of the extent of involvement of the white matter tracts. Using diffusion tensor imaging (DTI) several studies have shown damage to ventrorostral temporal white matter extending rostrally towards the frontal lobe and dorsocaudally towards the superior temporal and supramarginal gyri [104, 109, 110]. The major white matter tracks emanating from the anterior temporal lobe – the uncinate, arcuate, and inferior longitudinal fasciculi – are also abnormal. In the case of the former two bundles, the changes extend well beyond the rostral temporal lobe; but abnormalities of the inferior longitudinal bundle appear not to extend caudal to the atrophic/hypometabolic zone [104]. What is most striking about SD is the coherence of findings in regional brain abnormality whether this is assessed in gray matter, white matter or by metabolic measures [19, 104, 108, 111, 112]. The concordance between atrophy and hypometabolism in SD (illustrated in Figure 12.3) is in striking contrast to the disjunction seen in early Alzheimer’s disease, where parietal association regions reveal only minor atrophic changes but severe hypometabolism [113].
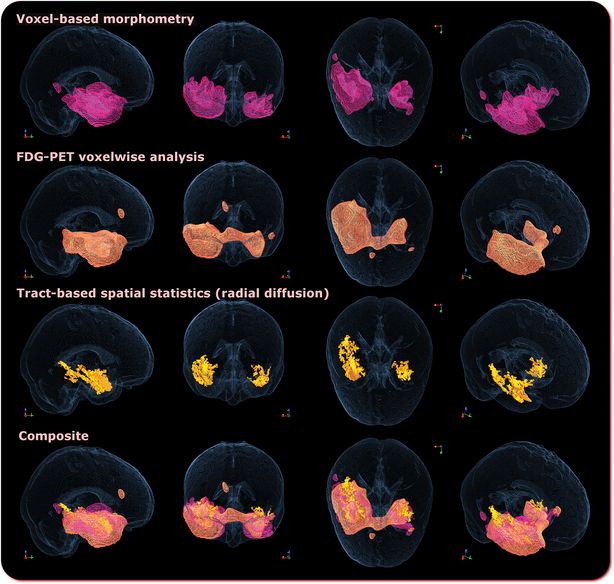
Comparison of regional gray matter atrophy on MRI (row 1); reduced mean 18F–2-fluoro-2-deoxy-d-glucose-PET (row 2); increased radial diffusivity (row 3); and a composite of the first three (row 4) for ten patients with semantic dementia compared with 21 healthy controls, all at family-wise error-corrected P < 0.05. Note the co-localisation of atrophy and hypometabolism.
As well as hippocampal atrophy, patients with SD show hypometabolism of the medial temporal lobe indistinguishable from that seen in AD [111]. One critical difference, however, is that in AD the hypometabolism extends to other components of Papez’s circuit (mammillary bodies, dorsomedial thalamus, and posterior cingulate), whereas in SD these other interconnected elements are preserved which may be fundamental to the relative sparing of episodic memory in SD [111].
Genetics
The strength of family history differs across the variants of FTD and PPA and appears to be lowest in SD. For instance in our study of 100 patients [114], there was a family history of “dementia” in 15 but only two patients had a clear family history of early onset dementia or “Pick’s disease.” There were no families with more than two affected members. The familial rate was estimated, therefore, at between 2% and 7%.
Mutations of either the MAPT, the progranulin (GRN) or the C9ORF72 gene can now be found in the majority of FTD cases with an autosomal dominant pattern of inheritance. The latter seems to be the most common and is particularly linked to familial FTD-MND but is also found in patients with bvFTD, some of whom may have a family history of MND [115]. Despite being associated with FTLD-TDP (see below), this mutation appears to be very rare in patients with SD. In a recent study in which 114 patients with one of the clinical phenotypes of FTD or PPA were screened for the C9ORF72 mutation, none of those with SD were found to carry the mutation [116].
Neuropathology
The neuropathology of the frontotemporal dementias is a complex and rapidly evolving subject and is dealt with in detail elsewhere in this book. In brief, cases share, by definition, the finding of bilateral frontotemporal atrophy with neuronal loss, microvacuolation, and a variable degree of astrocytic gliosis.
The subtypes of underlying pathology in patients with FTD are classified on the basis of the pattern of protein accumulation and are referred to collectively as frontotemporal lobar degeneration (FTLD) [117, 118]. In SD the predominant pathology is FTLD-TDP [119, 120]. In our report of 100 patients from Cambridge given a clinical diagnosis of SD, pathological confirmation was available in 24 of the 32 deceased cases: 18 (72%) had FTLD-TDP, three had classic tau-positive Pick body FTD (FTLD-tau), and three had Alzheimer pathology [114]. Those with FTLD-tau and AD pathology appeared clinically identical to those with FTLD-TDP pathology.
Prognosis
The prognosis for FTD is quite variable from patient to patient. Earlier studies from Cambridge suggested a rather rapid course for SD but, with increased experience, it became apparent that many patients survive for over a decade from diagnosis. In our study of 100 patients, a Kaplan Meier survival analysis of the whole group (Figure 12.4) indicated a median survival of 12.8 years (95% confidence interval = 11.9 to 13.7). A number of analyses failed to identify factors clearly predicative of outcome including pattern of atrophy at presentation and, surprisingly, severity of dementia at presentation as judged by score on the Mini Mental State Examination or Clinical Dementia Rating Scale, naming performance on the semantic battery, or total Neuropsychiatric Scale score [114].
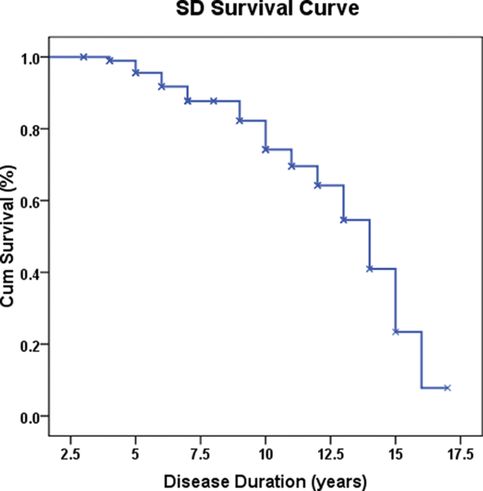
Cumulative survival of 100 SD patients. Disease duration is given in years.
Management
At present, no treatments are available to either reverse or halt the course of the disease. Randomized controlled trials of drugs, such as memantine, have unfortunately proven ineffective to treat cognitive symptoms [121]. A small, but growing series of case reports, however, support the use of cognitive interventions to remediate word retrieval impairments, at least in the short term [122]. While only the specific words trained show improvement through these techniques, and ongoing intervention is needed to maintain the benefits, medium to strong effects have been shown in both mild and severely impaired patients [123]. Relearning appears particularly effective for items for which there is still partial comprehension [124], using active, but errorless learning techniques which pair a photograph of each object with the associated word [125, 126]. Improvements are typically observed within a matter of weeks, and the intervention can be completed in the home environment using simple computer programs [123, 125–128].
As only trained words seem to improve, the selection of words is an important consideration and should relate to the patient’s everyday functional and communication needs. Encouragingly, patients can generalize their knowledge as long as exemplars are not too visually dissimilar [125, 127], with improvements reported in both expressive and receptive language skills, particularly in the earlier stages of the disease [129].
To maintain the benefits of training, patients require continued practice [130]. However, in the absence of alternative therapies, such interventions provide relief to functional language problems in everyday living and a sense of empowerment in an otherwise declining landscape.
Significant behavioral changes typically accompany the cognitive deficits and are a significant cause of carer burden and stress [131–133]. The use of drug treatments to address mood or behavioral symptoms may be appropriate using selective serotonin-reuptake inhibitor or low-dose neuroleptic medication. In addition, carer education and support groups focused on problem-solving strategies and cognitive reframing may provide important assistance in coping strategies [134, 135] and thereby reduce carer burden. As with other forms of FTD the importance of education and ongoing caregiver support cannot be over-emphasized.
Concluding comment
Despite its relatively short history – scarcely more than two decades – intense research interest in SD has produced a rich and comprehensive picture of a coherent disorder. Although the disease is of course seriously distressing for the patients and their families, SD has proven dramatically informative about the organization and neural basis of one of the most central aspects of human cognition: semantic memory. It has also emerged as the most distinctive of the variants of FTD in terms of the clinical, neuropsychological, radiological, and pathological profile.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







