Chapter 13 Sinonasal Disease
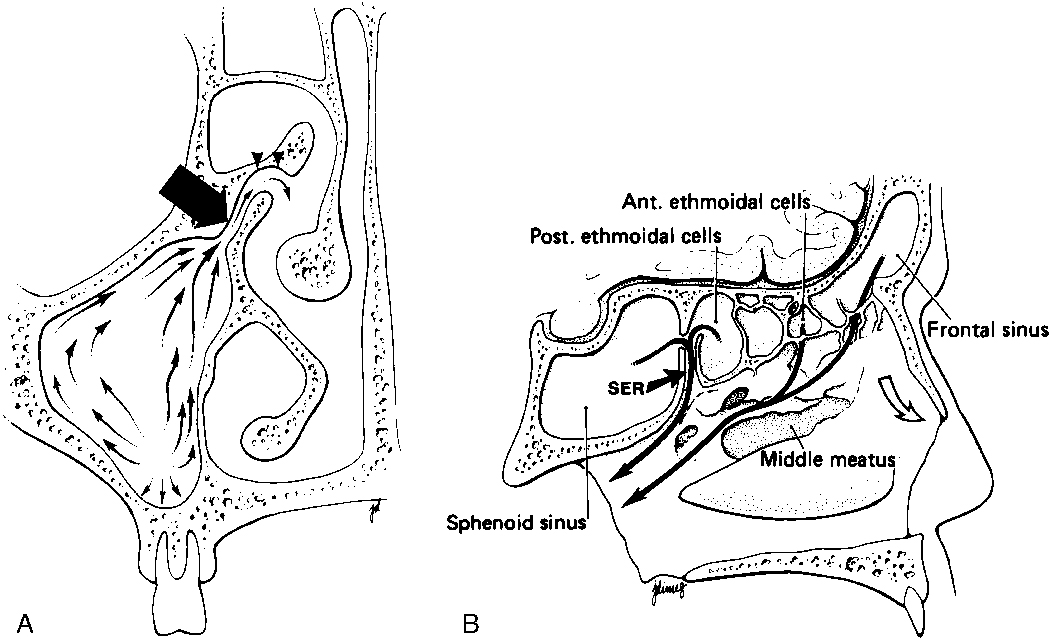
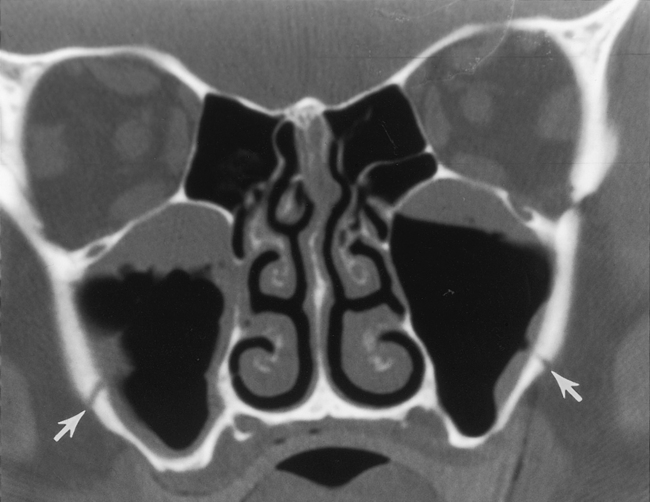
To appreciate the pathogenesis of sinusitis, you must understand the normal anatomic pathways of mucociliary clearance in the paranasal sinuses (Fig. 13-1). The cilia within the maxillary sinus propel the mucus stream in a starlike pattern from the floor of the maxillary sinus toward the ostium, situated superomedially. In approximately 30% of patients, a second accessory ostium to the maxillary sinus is present inferior to the major opening. From the maxillary sinus ostium, mucus from the maxillary antrum (the maxillary antrum and maxillary sinus are synonymous) gets swept superiorly through the infundibulum, which is located lateral to the uncinate process and medial to the inferomedial border of the orbit. The uncinate process, a sickle-shaped bony extension of the lateral nasal wall extending anterosuperiorly to posteroinferiorly, is rarely (<2.5% of patients) pneumatized itself. Occasionally, the uncinate process attaches to the lamina papyracea (the medial wall of the orbit). If it does so, the infundibulum does not have a superior opening, thus creating a blind pouch, the recessus terminalis. The hiatus semilunaris is a slitlike air-filled space anterior and inferior to the largest ethmoid air cell, the ethmoidal bulla, and right above the uncinate process. Mucus is passed through the hiatus semilunaris posteromedially via the middle meatus, a channel between the middle turbinate and the uncinate process, into the back of the nasal cavity to the nasopharynx, where it is subsequently swallowed.
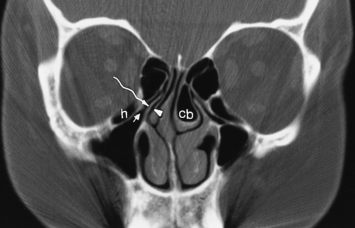
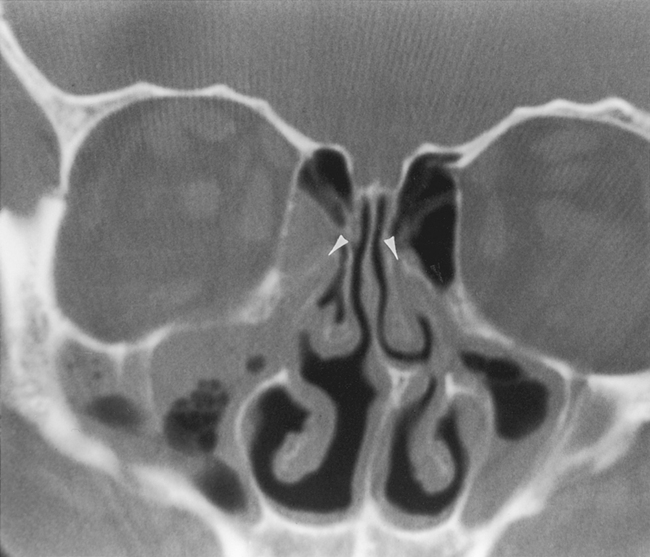
The most anterior ethmoid air cells located in front of the middle turbinate’s cribriform plate attachment are termed agger nasi cells. Agger nasi cells lie anterior, lateral, and inferior to the frontal recess. They are present in more than 90% of patients (Fig. 13-2). The roof of the ethmoid sinus is termed the fovea ethmoidalis.
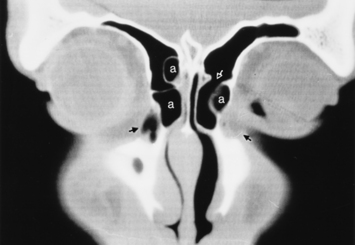
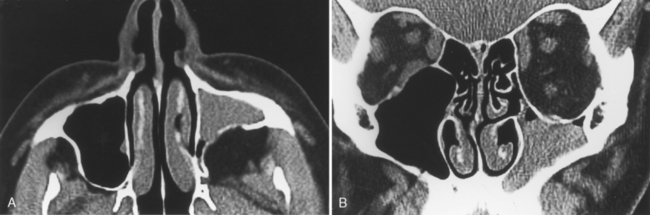
As stated earlier, ethmoidal bulla is the term used for the ethmoid air cell directly above and posterior to the infundibulum and hiatus semilunaris. A very large ethmoidal bulla can obstruct the infundibulum and hiatus semilunaris, leading to interference with the drainage of the maxillary and anterior ethmoid sinuses. When anterior ethmoid air cells are located inferolateral to the bulla, along the inferior margin of the orbit protruding into the maxillary sinus, they are termed Haller cells, maxilloethmoidal cells, or infraorbital cells. They are seen in 10% to 45% of patients. When greatly enlarged, Haller cells may narrow the infundibulum or maxillary sinus ostium (Fig. 13-3).
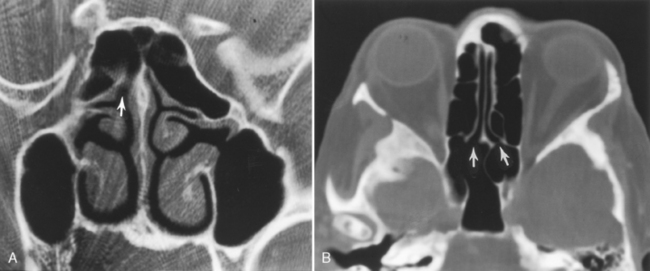
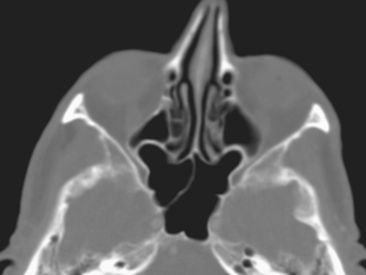
The posterior ethmoid air cells are located behind the basal (or ground) lamella of the middle turbinate and drain via the superior meatus, the supreme meatus, or other tiny ostia just under the superior turbinate. Ultimately, these ostia drain into the sphenoethmoidal recess of the nasal cavity (Fig. 13-4), from which the secretions pass to the nasopharynx. In some patients the most posterior ethmoid air cell may pneumatize into the sphenoid bone, superior to the sinus. This is termed an Onodi cell.
The nasolacrimal duct (see Chapter 10) courses downward from the lacrimal sac bordering the medial canthus, where it is in close association with agger nasi air cells. Inflammation of agger nasi cells may be associated with epiphora because of this close relationship. The duct subsequently runs in the anterior and inferior portion of the lateral nasal wall. Its ending opens below the inferior turbinate at the inferior meatus.
IMAGING MODALITIES
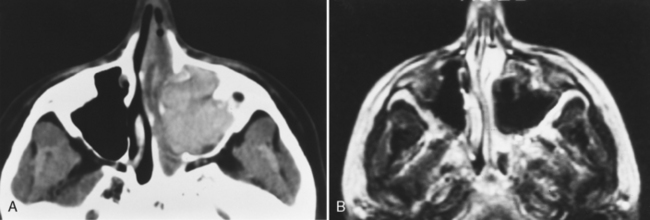
Magnetic resonance (MR) examination of the sinonasal cavity can be performed in a standard head coil or, for more precise anatomic resolution, with a surface coil placed over the anterior part of the face. Both T1-weighted images (T1WI) and T2-weighted images (T2WI) are required because of the variability of signal intensity of sinonasal secretions caused by protein concentration. Fat-suppressed enhanced T1WI is employed for the evaluation of complicated sinusitis or for suspected neoplastic disease. Differentiating tumors from infections of the sinonasal cavity may be best achieved with enhanced MR: Infected mucosa enhances in a peripheral fashion, whereas tumors usually enhance solidly and centrally (Fig. 13-5).
CONGENITAL DISORDERS
Choanal Atresia
Choanal atresia is usually diagnosed in infancy because neonates are obligate nose breathers as they suck on a bottle or breast (Box 13-1). The child is seen with respiratory distress. Although the diagnosis can be suggested by the inability to pass a nasogastric tube through the nose, imaging is necessary to determine whether the obstruction is membranous (15% of cases) or bony (85%) and whether other congenital central nervous system (CNS) or non-CNS anomalies are associated (50%). In addition to the narrowed posterior choana, look for thickening of the vomer (Fig. 13-6). The posterior choanal opening should be more than 0.5 cm wide in neonates, 1 cm in adolescents. The vomer should measure less than 0.34 cm in children under age 8 years. Rather than atresia, some patients have mere stenosis of the passageway. Often, unilateral choanal atresia may escape detection into adulthood. Patients may be unaware of the hyposmia often associated with this disorder.
Box 13-1 Entities Associated with Choanal Atresia
A dacryocystocele or piriform aperture stenosis may mimic choanal atresia clinically.
Dermoids, Sinus Tracts, and “Gliomas”
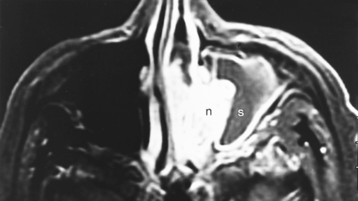
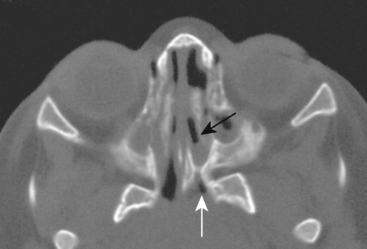
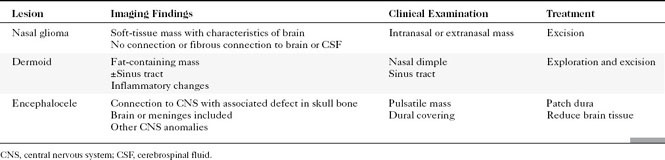
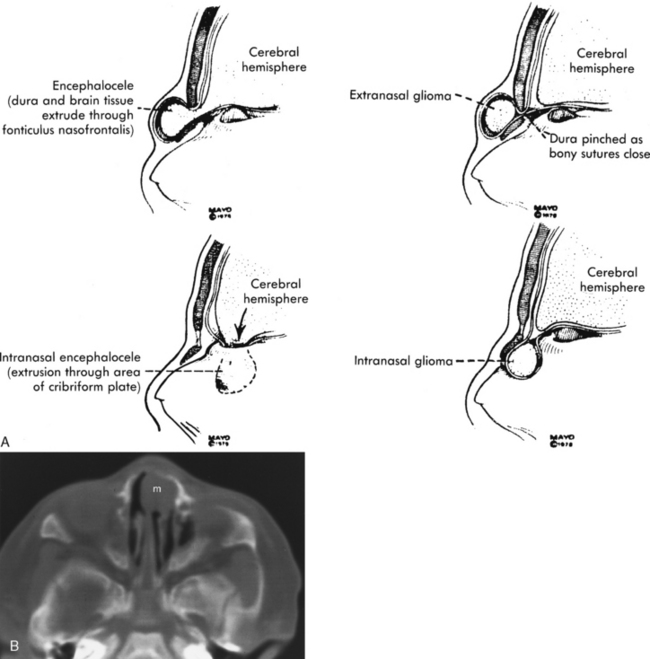
Congenital lesions of the sinonasal cavity include congenital encephaloceles, dermoid cysts, sinus tracts, and nasal gliomas (Table 13-1). These lesions occur as an abnormality in the process of invagination of the neural plate. In embryogenesis, the dura of the brain contacts the dermis at the nasion region as the neural plate retracts. Normally, the dermal connection regresses; when it does not, one of these lesions may develop. A cerebrospinal fluid (CSF) connection to the intracranial contents is maintained with meningoencephaloceles (see Chapter 9), whereas the connection is fibrous only with a nasal glioma (Fig. 13-7). Nasal gliomas are NOT neoplasms but congenital anomalies. They are extranasal more commonly than intranasal. (What an oxymoron—nasal gliomas are usually extranasal and are not gliomas.) Most patients with dermoid sinus tracts have a pit in the middle of the nose. Dermoid cysts occur more commonly than tracts; however, tracts may cause more severe symptoms because of their intracranial connection in 25% of cases. Thus meningitis, osteomyelitis, and intracranial abscesses may occur in the setting of dermoid tracts.
Hypoplastic Maxillary Antrum
Bony changes that suggest the diagnosis of a hypoplastic antrum are listed in Box 13-2. Causes of overexpansion of paranasal sinuses are listed in Box 13-3. In the differential diagnosis of sinus hypoplasia is the “silent sinus syndrome,” or maxillary sinus atelectasis. In this entity, ostial obstruction from chronic sinusitis leads to chronic negative pressure, which leads to hypoventilation, which, over time, reduces the sinus’ volume, hence sinus atelectasis. Patients present with enophthalmos (not sinus symptoms, strangely enough) as the orbital floor becomes depressed, the maxillary walls retract centripetally, and the retromaxillary fat fills the space left by the atelectatic sinus. The CT shows the retracted maxillary sinus walls in association with a small-volume, opacified sinus (Fig. 13-8).
INFLAMMATORY LESIONS
Sinusitis
Anatomic Considerations
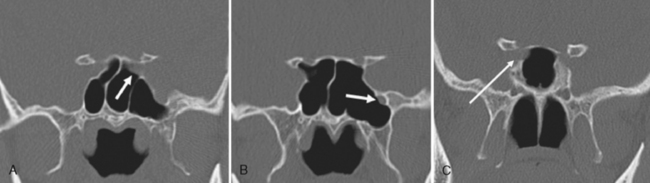
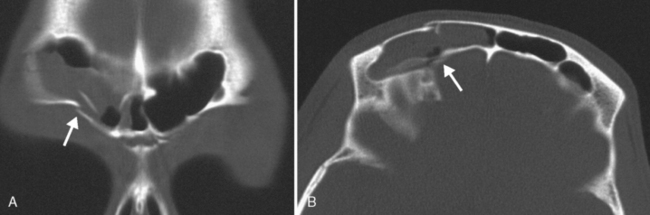
Are there areas of dehiscence in the cribriform plate and sphenoid sinus walls? Remember that there is an attachment of the middle turbinate to the cribriform plate. If the surgeon tugs too hard, the cribriform will fall and down will come CSF or brain through the new foramen in the skull base. The potential for intraorbital, intracranial, carotid, or optic nerve perforation at the time of surgery depends on these anatomic variants, found in 4% to 15% of patients. Three percent of people have optic nerves that are in contact with the posterior ethmoid wall—most course along (90%) or through (6%) the sphenoid sinus. An area of bony dehiscence seen on CT is present in 24% of optic nerves, putting it at risk (Fig. 13-9). There have been limited reports of optic nerve transection during sphenoethmoidectomy from an intranasal approach, and dehiscence of the sphenoid wall may be a predisposing factor. An intersinus septum in the sphenoid sinus that attaches to the carotid canal is important to recognize preoperatively and is typically best identified in the axial view (Fig. 13-10). Overvigorous removal of such an intersinus septum during surgery may result in carotid laceration.
Appearance on Imaging
OMC opacification correlates well with the development of sinusitis (Fig. 13-11). The positive predictive value of infundibular opacification for the presence of maxillary sinus inflammatory disease is approximately 80%. When the middle meatus is opacified, the maxillary and ethmoid sinuses show inflammatory change in 84% and 82% of patients, respectively. The specificity of middle meatus opacification for maxillary or ethmoid sinus disease is more than 90%. These findings support the contention that obstruction of the narrow drainage pathways leads to subsequent sinus inflammation.
The presence of air-fluid levels is more typically associated with acute sinusitis than with chronic inflammatory disease. In cases of suspected acute sinusitis, air-fluid levels or complete opacification of a sinus is present in 63% of cases (Fig. 13-12). Using these two criteria (air-fluid levels and complete opacification), CT has a 90% positive predictive value for acute sinusitis. Bubbles of air mixed with soft tissue in the sinus are another predictor of acuity. Of course, acute sinusitis may be superimposed on chronic changes. The findings suggestive of chronic sinusitis include mucosal thickening, bony remodeling, polyposis, mucus retention cysts, and bone thickening secondary to osteitis from adjacent chronic mucosal inflammation (Fig. 13-13). Some people exclude mucosal thickness of less than 3 mm as normal passive mucosal congestion that does not indicate inflammation, particularly in children.
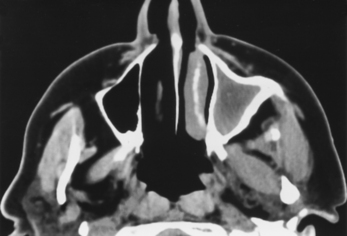
Figure 13-13 Chronic sinusitis. Marked bony thickening around the opacified left maxillary sinus signifies chronic disease.
Hyperdense secretions on CT may be due to six main causes: (1) inspissated secretions, (2) fungal sinusitis, (3) hemorrhage in the sinus, (4) polyps, (5) mucocele, and (6) calcification. The hyperdense sinus may be the only clue to fungal sinusitis and is an important feature to note. However, chronic sinusitis infected with bacteria occasionally is hyperdense on CT, particularly in patients who have very long-standing disease or cystic fibrosis. The hyperdense sinus often corresponds to the hypointense sinus on T2WI (Fig. 13-14). Nonetheless, say “a fungus is among us” if you see dense secretions.
Trauma often affects the walls of the paranasal sinuses. Remember that the floor of the orbit serves as the roof of the maxillary sinus, so fractures there cause blood levels in the sinuses. Similarly, the medial wall blowout fracture affects the ethmoid sinus, and orbital roof fractures may affect supraorbital ethmoid cells or the frontal sinus. Direct blows to the forehead may cause inward displacement fractures of the frontal sinuses (Fig. 13-15). Skull base fractures may cross the sphenoid sinus. These air-filled spaces are not necessarily the best buttresses against trauma, being very thin and with nothing but air between them and the directed force.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree