Fig. 1
T1-weighted MRIs with gadolinium showing central skull base meningioma with supero- and latero-sellar extensions and their relationships with the surrounding structures in coronal (a), axial (b), and sagittal (c) sections
Computed Tomography (CT)
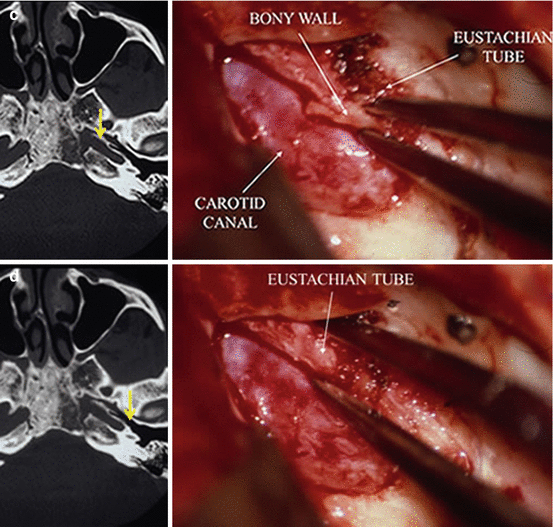
Thin section CT scan with bone windows is a useful, if not necessary, complement to MRI to delineate meningioma relationships with the neighboring bony structures. It should be systematically studied: anterior and posterior clinoid processes, sella turcica, orbital apex, floor of the temporal fossa, sphenoid wing and its foramens, and petrous apex. It should be systematically searched for invasion of the sphenoid sinus cavities and of the maxillary fossa and also extension to the following key basal structures: the optic canal; the superior orbital fissure; the foramens rotundum, ovale, and spinosum; the intrapetrous carotid canal, and the foramen lacerum (Fig. 2a–c). Sphenoid sinus variations that may complicate CS surgery, such as pneumatization of the anterior clinoid process, can easily be detected by CT scan (Fig. 2d). CT especially with 3D spiral CT reconstruction is prepicious to quantify eventual bony hypertrophy (Fig. 3a) or lysis or invasion of the anterior clinoid process (Fig. 3b) or other bony structures associated with cavernous sinus meningiomas (Fig. 3c). Hyperostoses, because they frequently correspond to tumor infiltration, will be the target of resective drilling [24].
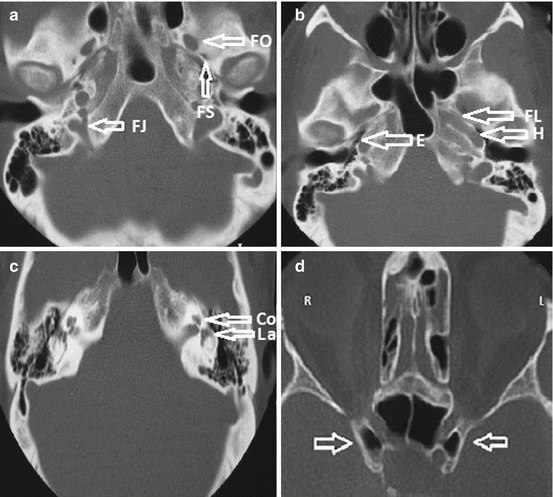
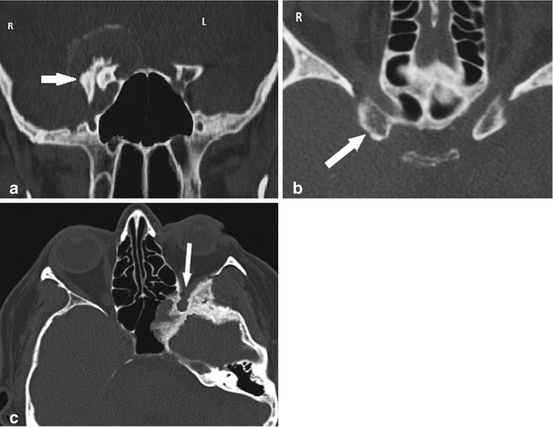

Fig. 2
Thin sections of CT scan with bone windows showing bone anatomy of middle cerebral fossa and petrous bone. (a) Foramen ovale (FO), foramen spinosum (FS), and foramen jugularis (FJ). (b) Horizontal portion of the intrapetrous carotid canal (H), Eustachian tube (E), and foramen lacerum (FL). (c) Cochlea (Co) and labyrinth (La). (d) Sphenoid sinus variations (in another patient) such as pneumatization of the anterior clinoid processes that might complicate surgery, namely, cerebrospinal fluid fistula after drilling (arrows)

Fig. 3
(a) Coronal CT scan with bone windows of a right CS and anterior clinoid meningioma showing hyperostosis of the anterior clinoid process with calcification at meningioma insertion (arrow). (b) Axial CT scan with bone windows of a right cavernous sinus and orbital apex meningioma showing hypertrophied and invaded anterior clinoid process (arrow). (c) Axial CT scan with bone windows of a left cavernous sinus and sphenoid wing meningioma showing hyperostosis and invasion of the superior orbital fissure and lateral wall of the sphenoid sinus (arrow)
Digital Subtraction Angiography (DSA)
Additional selective four-vessel cerebral DSA via femoral artery brings supplementary informations on the relation of the meningioma with the ICA: its segments and main arterial branches, as well as their degree of invasion (Fig. 4).
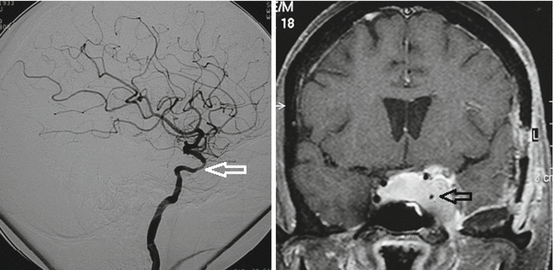

Fig. 4
Relationships of CS meningioma with ICA. Invasion and narrowing of left ICA and its C4 and C5 segments according to Bouthillier’s classification (arrows). Left: internal carotid artery DSA by femoral route in lateral view. Right: T1-weighted MRI with gadolinium in coronal section. According to Bouthillier’s classification, the first segment of the ICA is the cervical segment (C1) which begins at the level of the common carotid artery bifurcation and runs inside the carotid sheath and ends where it enters the carotid canal of the petrous bone; the second segment of the ICA is called the petrous segment (C2) which begins at the entrance of carotid canal and ends at the posterior edge of the foramen lacerum; the third segment is the lacerum segment (C3) which begins where the carotid canal ends and ends at the superior margin of the petrolingual ligament which runs between the lingula of the sphenoid bone anteriorly and the petrous apex posteriorly; the fourth segment of the ICA is the cavernous segment (C4) which begins at the superior margin of the petrolingual ligament and ends at the proximal dural ring; the fifth segment is the clinoid segment (C5) which begins at the proximal dural ring and ends at the distal dural ring; the sixth segment is the ophthalmic segment (C6) which begins at the distal dural ring and ends just proximal to the origin of the posterior communicating artery; the seventh segment is the communicating segment (C7) which begins just proximal to the origin of the posterior communicating artery and ends at the ICA bifurcation
DSA is the only mean to precisely evaluate the fine vascularization of the meningioma and identify its feeders. Feeding arteries arise in various ways from the ICA, its lacerum (C3), cavernous (C4), clinoid (C5), ophthalmic (C6), and communicating (C7) segments (according to Bouthillier’s classification) [5], and additionally, frequently, from the ophthalmic artery itself (Fig. 5a, b). Also feeders may come from the middle fossa branches of the external carotid artery (Fig. 5c). Therefore, a selective angiography is useful in studying independently the internal and the external carotid system. Selective DSA also gives opportunity for preoperative embolization of the tumor when the vascularization coming from the external carotid artery is significant. It also permits to evaluate the caliber of the several branches of the external carotid artery for choosing the best donor branch, in the eventuality of the need for an extra–intracranial arterial anastomosis.
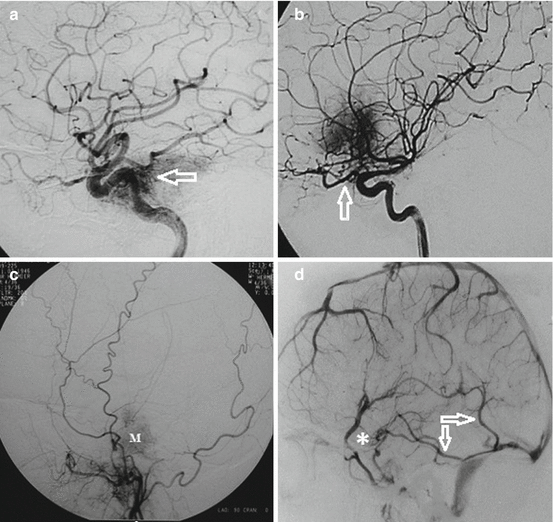

Fig. 5
Selective internal carotid artery (ICA) DSA from different patients (lateral views). (a) Selective ICA DSA showing feeding vessels of the meningioma from lacerum segment (C3) (arrow). (b) Selective ICA DSA showing feeding vessels of the meningioma from paraclinoid ICA and from ophthalmic artery through ethmoidal arteries (arrow). (c) Selective external carotid artery DSA showing feeding vessels of the meningioma (M) from the middle fossa branches of the external carotid artery. (d) Venous phase of DSA showing superficial sylvian veins draining anteriorly to the skull base (asterisk) and inferior cerebral veins draining to the lateral sinus via the veins of Labbé (arrows). Such venous drainages are at risk on cavernous sinus approach and surgery
Cerebral DSA gives the opportunity to appreciate the capacity of supply of the Willis circle in the eventuality of the necessity of carotid control: by the anterior communicating artery with compression of the contralateral carotid artery at the neck or by the posterior communicating artery from the vertebrobasilar system, with compression of the ipsilateral carotid artery at the neck during injection of the vertebral artery. In rare occasions, a carotid artery occlusion test with balloon may be performed in the eventuality of a therapeutic ICA occlusion.
Surgical Strategy
This complex of neuroimagings is the basis to deciding the surgical strategy, the type of craniotomy and complementary osteotomy, the usefulness of an anterior clinoidectomy and of a proximal control of ICA at the foramen lacerum, and also the necessity of an extra–intracranial arterial bypass.
Surgical approach should permit to have a working zone large enough with multiple angles for attacking the various extensions of the meningioma [34]. In addition, a large multi-angled approach allows to cut vascular supply from the petrous segment (C2), lacerum segment (C3), and paraclinoid and supraclinoid segments (C5 and C6) of the ICA according to Bouthillier’s classification [5]. We hypothesized that this strongly contributes to reduce regrowing of the meningioma by means of its devascularization. Also coagulation of the feeders coming from branches of the external carotid artery through the various foramens of the base participates to devascularization at its insertion in the middle cranial fossa.
Surgical approach should also be determined as such to escape from major morbidity due to excessive retraction of the cerebral parenchyma and sacrifices of the basal draining venous structures [32, 33].
Craniotomy/Osteotomy
Craniotomy is of the frontopterionotemporal type, centered over the sphenoid ridge (Fig. 6a). It is more extended to the frontal side when the meningioma has a predominant suprasellar extension, to the temporal side for cases with a predominant latero-sellar extension and/or extension to the tentorial incisura and the retro-clival region. Opening of the frontal sinus is avoided to prevent from secondary infection risk.
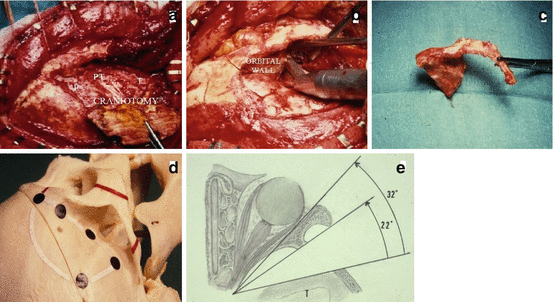

Fig. 6
Surgical technique. (a) Pterional craniotomy completed. (b) Removal with sagittal saw of the orbitozygomatic arch, together with the superolateral part of the orbital wall. (c) Orbitozygomatic bone after osteotomy. (d) Landmarks of bone flap and orbitozygomatic osteotomy (OZO). (e) Schematic representation of view angle gained by OZO (axial section); OZO gives additional 70 % exposure [1]. OZO facilitates exposure of the superior dome of the tumor when invaginated into the base of the brain (see Fig. 1) and gives different ways to attack the tumor according the concept of “cone of approach” [6]. OZO avoids opening the sylvian fissure and consequently reduces the risk of sacrificing the sylvian venous drainages and also prevents from surgical manipulations of the middle cerebral artery and branches. F frontal, PT pterional, T temporal
Additional osteotomy (generally orbitozygomatic) is performed when the height of the meningioma is important and its invagination into the base of the cerebrum is pronounced as shown in Fig. 1. Such features are best evaluated on MRI coronal sections. Osteotomy is done with sagittal saw as a second step after performing frontopterionotemporal bone flap (Fig. 6b, c). This osteotomy gives additional 70 % exposure (Fig. 6d, e) and helps to avoid excessive retraction [1].
The cerebral veins of the inferior venous cerebral system of Labbé may limit the subtemporal approach and obliterate pathways to the middle fossa and block approach to the tentorial incisura. These veins have to be recognized prior to surgery to prevent from cerebral infarction. The bony aperture and the way the dura is opened should prevent from stretching and of course avulsion of these veins and unnecessary retraction of the brain which could be infarcted. Knowledge of the venous circulation is a wise precaution prior to surgery [32, 33].
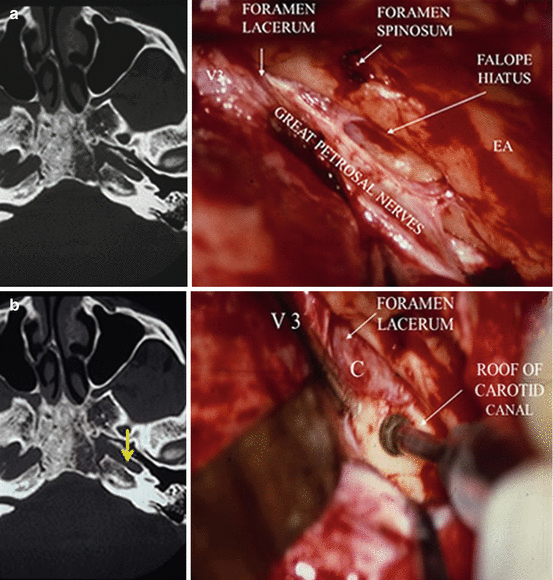
Extradural Approach to the Middle Cerebral Fossa and Proximal Control of ICA at the Foramen Lacerum and Adjacent Petrosal Canal
This part of the procedure is made easy by complementary orbitozygomatic osteotomy. Premier aim is to devascularize the tumor as possible as it can be. According to a recent study on 100 cases of cavernous sinus meningiomas, devascularization of the tumor was likely an important factor inhibiting regrowth of the tumor remnant. As a matter of fact, by surgery alone using this approach, only a minority of the meningiomas (13.25 %) had regrowth from the intracavernous remnant for a follow-up period of up to 20 years according to Kaplan–Meier statistical analysis [36]. Devascularization of the tumor is accomplished by coagulation and division of the middle meningeal artery at the foramen spinosum and also by coagulation with a thin bipolar forceps of the small branches of the external carotid artery which accompany the dural sleeves of the nerves on traversing their foramens at the cranial base, namely: the foramen ovale, foramen rotundum, and the superior orbital fissure (Fig. 7).
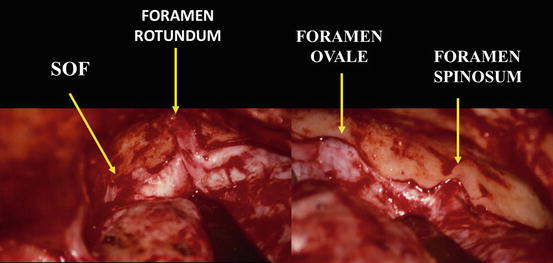

Fig. 7
Operative view of the floor of the middle cerebral fossa, from superior orbital fissure (SOF), anteriorly, to foramen spinosum and petrous bone, posteriorly. Right side, extradural exposure after OZO
Approach of the ICA is at the foramen lacerum, right at the posterior edge of the trigeminal V3 dural sheath before its penetration into the foramen ovale, then posteriorly by unroofing the adjacent portion of the petrous canal (Fig. 8). This permits reduction of the meningioma vascularization coming from the C2 (petrous) and C3 (lacerum) segments of the internal carotid artery (Fig. 8). In most cases, the ICA can be directly reached at the posterolateral border of the foramen lacerum, provided (strong) retraction anteriorly of the dural sheath of V3. Unroofing the carotid petrous canal with rongeur and drill from anterior to posterior should take care not to injure the Eustachian tube and the tensor tympani tendon, laterally, and above all not to open the cochlea by too extensive drilling, posteriorly. The benefit of proximal control of the precavernous ICA at the foramen lacerum is not limited to its contribution to devascularization of the meningioma. This procedure also allows to control the artery in the eventuality of a temporary clamping, in the exceptional situation of an accidental fissuration. Preoperative knowledge of the tumor vascularization, arterial relationships, as well as organization and functionality of the carotid supply from the Willis circle is crucial information.