Sleep and Internal Medicine
Susan M. Harding
Jeffrey W. Hawkins
INTRODUCTION
Sleep disturbances are common in many medical disorders. Medications used in the treatment of these disorders may disrupt sleep. This chapter reviews the clinical aspects of how medical disorders, including gastroesophageal reflux (GER), renal disease, fibromyalgia syndrome (FS), infectious diseases, selected endocrine disorders, chronic pain (CP) syndromes, and chronic fatigue syndrome (CFS), interfere with or alter sleep. Finally, sleep-associated medication side effects will be discussed.
SLEEP AND GASTROESOPHAGEAL REFLUX
Sleep’s Influence on Esophageal Physiology
Sleep influences esophageal function (1). Physiologic changes occur from wake to sleep, from sleep to wake, and from one sleep stage to another (2). The upper esophageal sphincter (UES) pressure decreases from 40 to 20 mm Hg with sleep onset and further decreases to 8 mm Hg during stable sleep, predisposing to aspiration (3). The lower esophageal sphincter (LES) is the primary antireflux barrier, and when the LES relaxes without a swallow, it is called a transient LES relaxation. Transient LES relaxations are the primary GER mechanism, accounting for 63% to 100% of GER episodes (4). Transient LES relaxations are confined to wake time and brief arousals, and so GER events occur primarily during arousals (5). Sleep also affects esophageal acid clearance. Esophageal acid clearance is markedly prolonged during sleep and requires an arousal (2,6). Furthermore, swallowing frequency is almost nonexistent during stable sleep, with swallowing occurring during brief arousals (3). Saliva production also ceases during sleep, impeding the ability to neutralize acid refluxate (7). Sleep facilitates proximal acid migration up the esophagus so that when a GER event occurs, there is risk of aspiration (8). Basal gastric acid secretion (not related to eating) has a circadian rhythm that peaks between 8:00 PM and 1:00 AM (9). Sleep also delays gastric emptying by disrupting gastric myoelectric function, thus predisposing to GER (10). Esophageal function during sleep predisposes to the development of esophageal and extraesophageal manifestations of GER.
Manifestations of Sleep-related Gastroesophageal Reflux
GER manifestations during sleep include sleep-related GER, sleep-related asthma, and sleep-related laryngospasm. Furthermore, GER frequently occurs in patients with obstructive sleep apnea (OSA).
Sleep-related Gastroesophageal Reflux
Sleep-related GER is included in the International Classification of Sleep Disorders-2 (11). GER occurring during sleep time is more injurious than that occurring in the diurnal condition. It is associated with more severe esophagitis and may play a role in the development of Barrett’s esophagitis and esophageal adenocarcinoma (12). Sleep-related GER is common, with 10% of respondents to a random sample telephone survey reporting nocturnal GER (13). In a Gallup survey of 1,000 adults with heartburn at least weekly, 79% of responders reported nighttime heartburn, 75% reported that it negatively impacted their sleep, and 40% noted impaired daytime functioning (14). In another study, predictors of sleep-related GER in the participants of the Sleep Heart Health Study included carbonated drink consumption, snoring, higher body mass index (BMI), insomnia, benzodiazepine use, daytime sleepiness, hypertension, and asthma (15). Sleep-related
GER occurs primarily during the first 2 hours of the sleep period (16). Also, the use of zolpidem significantly increased acid GER during sleep in the subjects randomized to zolpidem (17). Furthermore, GER events did not cause arousals, and so esophageal acid clearance was markedly prolonged because of this lack of an arousal response (17).
GER occurs primarily during the first 2 hours of the sleep period (16). Also, the use of zolpidem significantly increased acid GER during sleep in the subjects randomized to zolpidem (17). Furthermore, GER events did not cause arousals, and so esophageal acid clearance was markedly prolonged because of this lack of an arousal response (17).
Sleep-related GER causes excessive daytime sleepiness (EDS), impairs daytime functioning, and decreases work productivity (18). People with sleep-related GER have a lower quality of life (QOL) and more health care visits compared to people without sleep-related GER (18). Treatment of sleep-related GER has the potential to improve these outcomes. A multicenter, placebo-controlled trial of 750 adults with sleep-related GER noted that 6 weeks of esomeprazole 20 or 40 mg daily improved sleep quality and work productivity. A reduction in lost work hours was also noted (19). Table 35-1 reviews the potential consequences of sleep-related GER.
Sleep-related GER symptoms include multiple awakenings, substernal burning, and/or chest discomfort, indigestion, and/or heartburn. Other symptoms include a sour or bitter taste in the mouth upon awakening, regurgitation, water brash, coughing, and choking. Some patients may not have esophageal symptoms and present with EDS without an obvious cause.
Esophageal testing is not required to make the diagnosis of sleep-related GER (20). However, methods for detecting sleep-related GER include esophageal pH testing that has a sensitivity and specificity of approximately 90% (21). It can be integrated with polysomnography (PSG) for temporal correlation of sleep-related events. Esophageal pH testing is performed by placing an esophageal pH probe 5 cm above the LES. Many laboratories include dual pH probes, in which a proximal pH probe is placed at the UES or in the pharynx. Patients also record meal times, bedtime, and awakening times in a diary. An acid GER event is defined by the presence of material that has a pH of <4.0 (21). Figure 35-1 shows a GER
event associated with an arousal. Normative data are also shown in Table 35-2 (22). Diagnostic accuracy is based on data recorded over a 24-hour period. A wireless esophageal pH system is also available that sends data signals for >24 hours. Currently, this system cannot be integrated with PSG systems (23). Recently, the use of esophageal pH combined with impedance monitoring can assess both acid and nonacid GER events. This test is helpful in difficult cases, especially when patients have continued GER symptoms despite the use of aggressive GER therapy, including proton pump inhibitors (PPIs) (24).
event associated with an arousal. Normative data are also shown in Table 35-2 (22). Diagnostic accuracy is based on data recorded over a 24-hour period. A wireless esophageal pH system is also available that sends data signals for >24 hours. Currently, this system cannot be integrated with PSG systems (23). Recently, the use of esophageal pH combined with impedance monitoring can assess both acid and nonacid GER events. This test is helpful in difficult cases, especially when patients have continued GER symptoms despite the use of aggressive GER therapy, including proton pump inhibitors (PPIs) (24).
TABLE 35-1 IMPAIRED OUTCOMES WITH SLEEP-RELATED GER | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
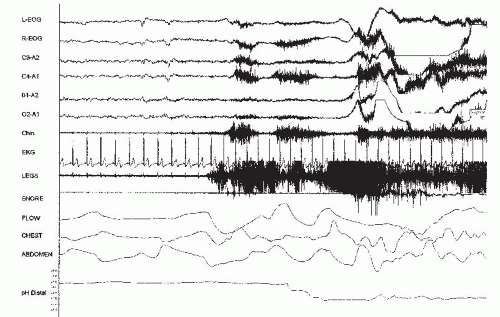 FIGURE 35-1 Thirty-second epoch showing esophageal pH integrated with PSG. The pH probe was placed 5 cm above the LES. Note the drop in esophageal pH to <4 during an arousal. |
Sleep-related Asthma
GER is present in approximately 50% of asthmatic patients (25). GER during sleep alters airway activity (26). Cuttitta et al. noted that GER episode duration correlated with increases in respiratory resistance (27). Sleep-related GER is also a risk factor for future asthma development. In the 5- to 10-year follow-up study of the 16,191 participants of the European Respiratory Health Survey, sleep-related GER was independently associated with future asthma development (28). Treatment of GER has the potential to improve airway function in asthmatic patients. In a multicentered double-blind, placebo-controlled trial using esomeprazole 40 mg twice daily for 16 weeks in 770 asthmatic patients, esomeprazole improved peak expiratory flow rates in asthmatic patients with nighttime asthma and GER symptoms, and so GER treatment has the potential to improve asthma during sleep in selected asthmatic patients (29).
Sleep-related Laryngospasm
GER may be important in sleep-related laryngospasm especially if refluxate traverses the UES and enters the larynx. Patients with sleep-related laryngospasm report an abrupt awakening with an intense feeling of suffocation often accompanied with stridor and choking sensations (1). Other symptoms include intense anxiety, a rapid heart rate, the sensation of impending death, and hoarseness. The differential diagnosis for sleep-related laryngospasm includes OSA, epilepsy, sleep-choking syndrome, sleep terrors, vocal cord dysfunction, and other upper-airway pathologies. Thurnheer et al. (30) noted that 9 out of 10 patients with sleep-related laryngospasm had GER documented by esophageal pH testing. Six patients responded to antireflux therapy, showing that GER may be associated with sleep-related laryngospasm.
Obstructive Sleep Apnea
Sleep-related GER symptoms are present in 62% of patients with OSA (31). Furthermore, 68% of consecutive patients with OSA have esophageal pH tests consistent with GER (32). Also, GER may be clinically silent in patients with OSA. Abnormal esophageal acid contact times were noted in 66% of patients with OSA who did not have GER symptoms (33). This possible link between GER and OSA is also noted in population studies. In the European Community Respiratory Health Survey of 2,202 young adults, participants who had sleep-related GER symptoms at least once weekly were more likely than those without sleep-related GER symptoms to report snoring (p < 0.001) and apnea symptoms (p < 0.01) (34).
However, this association is not a simple one. In >1,000 patients referred for OSA evaluation, GER symptom scores did not correlate with OSA variables (35). Another investigation in 136 patients referred for OSA evaluation noted no relationship between the Heartburn Severity Index score and the apnea-hypopnea index (AHI) (36). No relationship was found between esophageal pH data and AHI in 68 consecutive obese patients undergoing esophageal pH testing and PSG (37). However, another study of 94 consecutive patients being evaluated for OSA noted that patients with GER symptoms had a higher AHI than those without GER symptoms (p < 0.04) (38). An association was noted between erosive esophagitis and OSA severity. In 57 OSA patients with GER, logistic regression analysis noted a positive correlation between erosive esophagitis severity and AHI (p = 0.16) (39). Linking individual GER and OSA events on a temporal basis suggests that arousal is not the primary cause of GER events and that most OSA events do not induce GER events (40). Other investigators were also unable to find a temporal association between individual OSA and GER events (41,42).
There are many potential mechanisms of interaction between GER and OSA, including common risk factors for disease development, including obesity and alcohol use (43,44), and a common site of end-organ tissue injury including the pharynx and upper airway (45). Intrathoracic pressure changes during apnea events could result in insufficiency of the LES barrier and gastric cardia; however, one study noted the lack of a relationship between esophageal pH and esophageal pressure (thoracic pressure) (46). Multiple coexisting mechanisms may be active.
Although causality is difficult to prove, treatment of OSA can impact GER outcomes. In 331 patients with OSA, continuous positive airway pressure (CPAP)-compliant patients had a 48% reduction in sleep-related GER symptoms (p < 0.001) (31). A strong association was noted between higher CPAP pressures and sleep-related GER symptom improvement (r = 0.70, p < 0.001). Noncompliant CPAP patients did not have sleep-related GER symptom
improvement (31). Nasal CPAP also decreases esophageal acid contact times (47). Nasal CPAP increases LES pressure and esophageal gastric pressure, thus preventing GER events (48). In a small study, upper airway surgery for OSA reduced esophageal acid contact times 6 months postoperatively. A correlation was noted between improved AHI and arousal index, and reduction in esophageal acid contact times (r = 0.607, 0.730, p < 0.001) (49,50). Small uncontrolled studies examined whether GER treatment with PPIs improves AHI (32). There may be a possible effect, but more investigations are needed with placebo-controlled trials before a conclusion as to whether medical GER therapy improves OSA (32) can be arrived at.
improvement (31). Nasal CPAP also decreases esophageal acid contact times (47). Nasal CPAP increases LES pressure and esophageal gastric pressure, thus preventing GER events (48). In a small study, upper airway surgery for OSA reduced esophageal acid contact times 6 months postoperatively. A correlation was noted between improved AHI and arousal index, and reduction in esophageal acid contact times (r = 0.607, 0.730, p < 0.001) (49,50). Small uncontrolled studies examined whether GER treatment with PPIs improves AHI (32). There may be a possible effect, but more investigations are needed with placebo-controlled trials before a conclusion as to whether medical GER therapy improves OSA (32) can be arrived at.
So, in conclusion, data suggest that sleep-related GER is prevalent in patients with OSA, that mechanisms of interaction between the two disease states are complex, and that treatment of OSA with CPAP improves sleep-related GER variables.
Therapy for Sleep-related Gastroesophageal Reflux
All patients should be educated about lifestyle modifications (1). Patients should not eat for at least 2 hours before bedtime and avoid foods that promote GER, including high fat-containing foods, caffeine, chocolate, mint, alcohol, tomato products, citrus, and sodas. Medications that promote GER should be avoided if possible, including calcium channel blockers, anticholinergic medications, theophylline, prostaglandins, and bisphosphonates. Smoking significantly decreases LES pressure, so all patients should be encouraged to stop smoking. Patients should lose weight if they are obese and sleep in loose-fitting clothing.
Positional therapy can also be useful. Sleeping with the head of the bed elevated 6 inches by placing a full-length wedge or blocks under the head of the bed reduces GER. Recently, Khoury et al. (51) noted that sleeping in the right lateral decubitus position was associated with higher esophageal acid contact times. The left lateral decubitus position resulted in significantly lower esophageal acid contact times and appears to be the best sleeping position for sleep-related GER. A recent systematic review noted that positional therapy alone is inadequate and should be combined with other approaches (20).
Medical therapy includes antacids for acute symptom control, H2 receptor antagonists, PPIs, and prokinetic agents. H2 receptor antagonists provide heartburn relief in 60% of patients and can be given prior to sleep onset. PPIs provide superior gastric acid suppression. Dosing of PPIs should be 30 to 60 minutes before a meal, since these agents inhibit gastric acid secretion in actively secreting parietal cells. When examining optimal dosing schedules of omeprazole, Kuo and Castell (52) noted that giving 40 mg of omeprazole before dinner, or 20 mg before breakfast and dinner, resulted in better gastric acid suppression than giving 40 mg before breakfast only. Data show that there is nocturnal acid secretion despite PPI therapy (53). Whether this nocturnal gastric acid breakthrough is clinically important is not known.
Metoclopramide is the only prokinetic agent available for use in the United States, and it has a high prevalence rate (20%-50%) of central nervous system (CNS) side effects (54). Metoclopramide has a Food and Drug Administration (FDA) black box warning since tardive dyskinesia is potentially nonreversible, and so its use is not recommended (54).
Baclofen, which targets gamma-aminobutyric acid B (GABA-B) receptors (nonbenzodiazepine medications for sleep including zolpidem bind to GABA-A receptors), inhibits transient lower esophageal relaxations (55). Baclofen has many side effects that limit its usefulness in sleep-related GER. Hopefully, future medications will target motility mechanisms and be useful for the treatment of both acidic and nonacidic sleep-related GER.
Antireflux surgery, primarily fundoplication (both open and laparoscopic methods), is successful in 80% to 90% of patients. However, long-term results show that 62% of surgically treated patients use antireflux medications regularly (56). This data imply that surgery does not always replace the need for antireflux medication.
Clinical Pearls
Sleep-related GER occurs in 10% of the population and in 79% of persons who suffer heartburn weekly.
Symptoms include awakening with heartburn and/or regurgitation but may be clinically silent, with patients presenting with EDS without an obvious cause.
Esophageal pH testing is not required; however, esophageal pH testing integrated with PSG with or without esophageal impedance can be useful in diagnosing sleep-related GER.
Sleep-related GER can also result in sleep-related laryngospasm, worsening airway reactivity in asthmatic patients, and is prevalent in patients with OSA.
Therapy for sleep-related GER includes conservative therapy, positional therapy (head of bed raised or left lateral decubitus), and weight loss if obese.
PPIs have superior gastric acid suppression compared with other medications and should be given approximately 30 to 60 minutes before meals.
Patients who have undergone fundoplication may still require antireflux medications postoperatively.
Nasal CPAP improves sleep-related GER symptoms and esophageal acid contact times in patients with OSA.
SLEEP DISTURBANCES IN PATIENTS WITH RENAL DISEASE
Sleep disturbances are also very common in patients with renal disease. Most investigations include patients
with end-stage renal disease (ESRD) who are on chronic hemodialysis (HD) or continuous ambulatory peritoneal dialysis (CAPD). Sleep complaints occur in up to 80% of dialysis patients (58). Holley et al. (59) reported that the most common sleep complaints were nighttime awakenings in 67%, early morning awakenings in 80%, restless legs syndrome (RLS) in 72%, jerking legs in 83%, and daytime sleepiness in 28% of patients. Many patients napped more than an hour daily (59). Walker et al. (60) noted that 83% of patients had sleep-related complaints, with daytime sleepiness being the most commonly reported symptom (66%), followed by RLS (57%). Hays et al. (61) found that trouble sleeping and daytime sleepiness were in the top 12 of most bothersome symptoms in patients with renal disease. Sleep-related symptoms can be very stressful to patients and impair their QOL (61).
with end-stage renal disease (ESRD) who are on chronic hemodialysis (HD) or continuous ambulatory peritoneal dialysis (CAPD). Sleep complaints occur in up to 80% of dialysis patients (58). Holley et al. (59) reported that the most common sleep complaints were nighttime awakenings in 67%, early morning awakenings in 80%, restless legs syndrome (RLS) in 72%, jerking legs in 83%, and daytime sleepiness in 28% of patients. Many patients napped more than an hour daily (59). Walker et al. (60) noted that 83% of patients had sleep-related complaints, with daytime sleepiness being the most commonly reported symptom (66%), followed by RLS (57%). Hays et al. (61) found that trouble sleeping and daytime sleepiness were in the top 12 of most bothersome symptoms in patients with renal disease. Sleep-related symptoms can be very stressful to patients and impair their QOL (61).
Not only do dialysis patients have a high prevalence of sleep complaints, but they also have alterations in their sleep architecture. PSG findings in dialysis patients include reduced total sleep time (TST), sleep efficiencies as low as 66%, and large amounts of wake time after sleep onset (58). Minimal data are available as to whether improving sleep hygiene improves sleep architecture.
Many factors can impact sleep—wake mechanisms in patients with ESRD, including primary sleep disorders, depressed melatonin secretion, core body temperature elevation as a result of heat load from the dialysis bath, dialysis shift, napping, medications, noise from the dialysis machines, and fluid shifts (58,62,63).
The prevalence of OSA is higher in patients with renal disease compared with the general population. Kimmel et al., performing PSG in 30 patients with chronic renal failure, found that 73% of patients had sleep apnea (64). Chronic CAPD patients had increased sleep fragmentation and lower oxygen saturations from apneas on nights when fluid was present in their abdomens (65). An important study compared thrice-weekly HD patients with matched controls from the Sleep Heart Health Study. HD patients had more severe sleep-disordered breathing and nocturnal hypoxemia than the controls that was independent of age, BMI, and chronic disease status (66). Several mechanisms have been proposed to explain the development of OSA in patients with renal disease. Accumulation of uremic toxins affects upper airway muscle tone through CNS regulation during sleep (58). Edema and volume overload may also predispose the upper airway to collapse (67). Furthermore, hypocapnia from metabolic acidosis may change the apnea threshold, predisposing to an unstable breathing pattern. Protein metabolism may also play a role, since a diet rich in branched-chain amino acids results in a lower apnea index (58). Confounding factors, including age, may be important since both sleep apnea and ESRD are more prevalent in older people. There is evidence showing that aggressive therapy of ESRD improves sleep apnea. For instance, case studies show that sleep apnea was no longer present after kidney transplantation (68). A decrease in apnea index was also noted with the use of a bicarbonate versus an acetate-based dialysate. Furthermore, Hanly and Pierratos (69) reported that switching patients to slow nocturnal HD (8-10 hours a night) for up to seven nights weekly compared to HD for 4 hours three times weekly resulted in a marked decrease in the number of respiratory events. Also, conversion from conventional to nocturnal HD resulted in a decrease in ventilatory sensitivity to hypercapnia during hyperoxia in those patients who had a reduction in AHI with the change to nocturnal HD (70). However, this change in ventilatory sensitivity correlated with the AHI change in all subjects (70).
There is also an association between sleep apnea and early stages of chronic kidney disease (CKD). In a cross-sectional study, the probability of having sleep apnea in those with CKD (estimated glomerular filtration rate >45 mL/min per 1.73 m2) was sustained after controlling for hypertension, diabetes, and heart failure (71). Although this study did not establish causality, detection and management of sleep apnea may have the potential to improve the course of CKD (71). Clinical implications from these investigations suggest that all patients with renal disease should be screened for sleep apnea, and, if present, treated.
Patients with renal disease also have a high prevalence of RLS and periodic limb movement disorder (PLMD). Uremia is considered a secondary cause of RLS (72). Up to 80% of patients with RLS also have periodic limb movements during sleep (73). RLS is extremely distressing and occurs in approximately 80% of dialysis patients. Correction of anemia with erythropoietin reduces periodic limb movement frequency and improves sleep quality and daytime alertness. In patients with iron deficiency and ESRD, intravenous iron improved RLS and periodic limb movement syndrome (PLMS) in a placebo-controlled trial (74). (Further discussion is found in Chapter 15.)
Patients with ESRD also have EDS. Stepanski et al. (75) noted that 77% of questioned patients with CAPD reported taking daytime naps and 51% reported falling asleep unintentionally. A subset of their patient population underwent multiple sleep latency test (MSLT) showing a mean sleep latency (SL) of 6.3 minutes, verifying pathologic daytime sleepiness. EDS is also found in HD patients. It is very difficult to address the underlying causes of this condition in these patients, especially with the high prevalence of OSA, PLMS, and RLS. Other potential causes of EDS include uremic encephalopathy, parathyroid hormone excess (which could have neurotoxic effects), and alterations in neurotransmitter levels (58). Dialysis may also release cytokines that have somnogenic properties, including interleukin-1 (IL-1) and tumor necrosis factor alpha (TNFα) (76). Rapid changes in the acid—base balance and serum osmolality may also affect alertness. Furthermore, extrinsic factors may contribute to sleep disturbances as previously noted. In patients with less severe renal disease, nocturia may also disrupt sleep.
Clinical Pearls
Sleep complaints are present in approximately 80% of dialysis patients.
PSG features of dialysis patients include poor sleep efficiency with significant wake time.
OSA is prevalent in patients with renal disease.
Uremia is a secondary cause of RLS and PLMD.
Patients with renal disease often have EDS.
Patients with renal disease should be assessed for the presence of sleep disorders and, if present, aggressively treated.
FIBROMYALGIA SYNDROME
Clinical Features
FS is defined by the American College of Rheumatology as the presence of widespread musculoskeletal pain for at least 3 months, which is bilateral above and below the waist, including axial pain and the presence of 11 of 18 tender points (77). Note that fibromyalgia is a syndrome and not a disease and that the definition itself does not include a sleep disturbance. Investigators noted that adding a sleep disturbance to the definition did not improve the definition’s operating characteristics. FS is very common, affecting between 6 and 10 million Americans. In population-based studies, FS is thought to affect 3% of the population and is most prevalent in people aged 30 to 50 years (78). Interestingly, 75% to 90% of people with FS are women. Furthermore, health-seeking behavior is related to psychiatric diagnoses and not to FS itself. Pain symptoms may be severe, but remain stable over years. Factors associated with symptom onset include infection in 55%, physical trauma in 14% to 23%, and emotional trauma and stress in 14% of patients with FS. About 20% to 50% of patients with FS also have depression, and 53% to 65% of patients with FS have a history of sexual abuse (79). A meta-analysis and systemic review showed no significant association between sexual abuse and lifetime diagnosis of FS (80). Of the patients with FS, 23% have seasonal variations, with worse symptoms noted in December and January and fewer symptoms in July. There are other modulating factors reported in patients with FS. Poor sleep is an aggravating factor in 67% of patients, and rest is an ameliorating factor in 62% of patients (81).
Pathophysiology of Fibromyalgia Syndrome
The pathophysiology of FS is very complex. The main mechanism is thought to be central sensitization of nociceptive neurons in the dorsal horn of the spinal cord with activation of N-methyl-D-aspartate receptors (82). This central sensitization results in generalized heightened pain sensitivity due to pathological nociceptive processing within the CNS. There are changes in neurotransmitter systems, including substance P, noradrenaline, and serotonin, at the spinal cord and higher centers (83). A threefold increase in substance P and a decrease in serotonin levels are present in the cerebrospinal fluid (84). Opioid receptor regulation may also be important (85). There is evidence that patients with FS have functional polymorphism of the 5-hydroxytryptamine (5-HT) serotonin transporter gene. Increased inhibitory activities of the descending pathways by inhibition of serotonin and noradrenaline reuptake are mechanisms being explored in the treatment of FS (86). There are also alterations in endocrine function in patients with FS, including decreases in growth hormone (GH), insulin-like growth factor 1, free T4, and prolactin levels (87). Korszun et al. (88) reported that melatonin levels in patients with FS (compared with control subjects) are elevated between 23:00 and 06:50 hours.
There are alterations in regional cerebral blood flow (rCBF) in patients with FS, with lower rCBF to the thalamus and caudate nucleus (89). The thalamus plays a role in abnormal pain perception and processing, and the caudate nucleus is important for pain modulation. There is also decreased rCBF at cortical levels and subcortical nucleus (90). Gracely et al noted that pain is visible on functional magnetic resonance imaging scans. With pain, patients with FS had increased rCBF to 12 areas of the brain compared with 2 areas in the control subjects (91). These findings suggest that FS is characterized by both cortical and subcortical pain-processing augmentation. Furthermore, patients with FS have significantly less gray matter volume and a three times greater age-associated decrease in gray matter than matched controls. The longer period of time that patients with FS had FS, the greater the gray matter loss (92). Psychosocial and health status factors may also be important. The role of nonrestorative sleep is important in the idiopathogenesis of abnormal pain sensitivity in FS, which will be discussed now.
Sleep in Patients with Fibromyalgia Syndrome
Sleep is altered in patients with FS, with many patients having the alpha nonrapid eye movement (NREM) sleep anomaly. Sleep complaints are very prevalent, with 76% of patients with FS complaining of nonrestorative sleep, compared with 10% to 30% of control subjects (93). Patients report fragmented sleep and have sleep-onset and sleep-maintenance insomnia. They also associate pain with a prior night’s poor sleep (93,94).
There are also PSG findings. Based on seven controlled trials with 77 patients with FS, it was found that patients with FS had decreased TST, decreased delta sleep (adjusted for age), and decreased rapid eye movement (REM) sleep percentages. There were
more arousals, and long awakenings, and microarousals were three times more prevalent than in control subjects. The alpha NREM sleep anomaly was also noted (94). Sleep spindle frequency and spindle activity in 37 medication-free women with FS was reduced compared with matched controls, showing that thalamocortical mechanisms of spindle generation may be impaired in patients with FS (95).
more arousals, and long awakenings, and microarousals were three times more prevalent than in control subjects. The alpha NREM sleep anomaly was also noted (94). Sleep spindle frequency and spindle activity in 37 medication-free women with FS was reduced compared with matched controls, showing that thalamocortical mechanisms of spindle generation may be impaired in patients with FS (95).
Investigators examining the microstructure of sleep utilizing power spectral, frequency, and domain analysis in the electroencephalography (EEG) of patients with FS found that sigma frequency (12-14 Hz) and delta frequency (<2 Hz) reflected a sleep-protective function associated with greater depth; beta frequency (14-38 Hz) reflected arousal and was associated with microarousals and lightening of sleep; and occipital alpha frequency (8-13 Hz) was arousal associated (78). Interestingly, in normal control subjects, stage 4 sleep disruption by noise resulted in unrefreshing sleep, pain, and fatigue. Furthermore, in normal controls, deep joint pain stimulation reduced delta and sigma power and increased alpha and beta power on EEG spectral analysis.
The Alpha NREM Sleep Anomaly
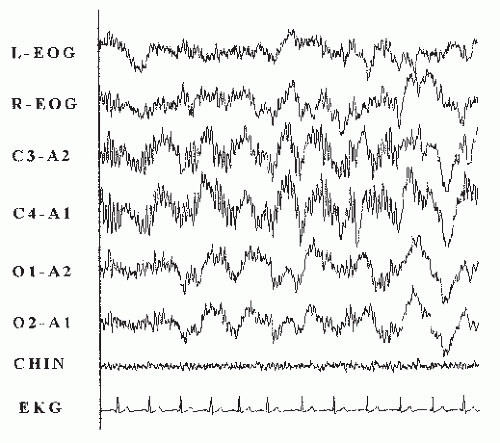
The alpha frequency (8-13 Hz) during NREM sleep was first observed in 1973. In 1975, Moldofsky (94) identified it in patients with FS, calling it “alpha intrusion,” and noted that it was frequently seen during delta sleep. This alpha NREM sleep anomaly is not specific for FS and not all patients with FS have it. Figure 35-2 shows a 10-second epoch of the alpha NREM sleep anomaly during delta sleep. Previous work has shown that the amount of alpha frequency correlates with overnight pain measures, mood, energy levels, and perceived shallow sleep. Branco et al. (96) noted that patients with FS had more alpha in successive sleep cycles compared with control subjects. Furthermore, Drewes et al. (97) noted that the alpha frequency is more prominent in the frontal area, and so the alpha frequency may be more prominent on the central versus the occipital lead on routine PSG. Mahowald and Mahowald (98) stated that the alpha NREM sleep anomaly was not specific to fibromyalgia, was not a marker for fibromyalgia, was not associated with enhanced long-term or short-term memory during sleep, and was not associated with myalgic symptoms (98). He stated that this anomaly was found in 15% of 240 normal subjects and was found more frequently in people with undisturbed sleep (20%) than in those with disturbed sleep (8%). He raised the question whether the alpha frequency might actually be a sleep-maintaining process. To quote Mahowald, “Van Sweden’s conclusion that ‘alpha-delta or alpha sleep is still an atypical and aspecific sleep pattern with an unclear pathophysiology’ is well stated.”
Roizenblatt et al. (99) noted that patients with FS have different alpha pattern characteristics and that these alpha pattern characteristics may identify patients with FS into subcategories. Monitoring sleep microstructure using power spectral and frequency domain analyses in 40 patients with FS (who were off their medications for >4 weeks) and 43 controls, they noted worsening of morning pain symptoms in 72% of patients with FS compared with 12% of controls. Of patients with FS, 93% also had an increase in the number of tender points after the sleep period, and poor sleep quality was reported in 70% of patients with FS compared with none in controls. Alpha rhythm was noted during NREM in 70% of patients with FS and in 16% of controls. Furthermore, the investigators noted three distinct patterns of alpha activity: (a) Phasic alpha pattern activity in which alpha was episodic, occurring simultaneously with delta; this pattern was seen in 50% of patients with FS and in 7% of controls; (b) the second pattern was tonic alpha, which was continuously present throughout NREM, independent of delta activity, and seen in 20% of patients with FS and in 9% of controls; and (c) a low alpha pattern with minimal alpha, which was seen in 30% of patients with FS and in 84% of controls. Furthermore, the phasic alpha pattern was associated with decreased sleep efficiency, decreased delta sleep, the subjective feeling of superficial sleep, and longer pain duration and morning stiffness. This phasic alpha pattern distinction may be useful in future research to identify patients for specific therapeutic modalities. Chervin et al. (100) evaluated 15 women with FS and 15 agematched controls, noting that women with FS had more stage shifts (p = 0.04) but did not show differences in
other PSG measurements or MSLT outcomes. Alpha EEG power during stage N3 NREM sleep or alpha power during the other sleep stages did not differentiate FS from control subjects. Conclusions about the significance of the alpha NREM sleep anomaly are difficult to make at this time (100).
other PSG measurements or MSLT outcomes. Alpha EEG power during stage N3 NREM sleep or alpha power during the other sleep stages did not differentiate FS from control subjects. Conclusions about the significance of the alpha NREM sleep anomaly are difficult to make at this time (100).
Primary Sleep Disorders in Patients with Fibromyalgia Syndrome
Sleep-disodered Breathing and Restless Legs Syndrome
Sleep-disordered breathing is prevalent in patients with FS. Shah et al. evaluated 23 patients with FS and 83% had an AHI of >15 (101). Gold et al. (102) noted that 27 out of 28 patients with FS had sleep-disordered breathing, with most having findings of inspiratory flow limitation with arousals consistent with upper airway resistance syndrome (UARS). Patients with FS should be screened for sleep-disordered breathing, including UARS.
RLS is also prevalent in female patients with FS. Stehlik et al. (103) examined 342 female patients with FS for the presence of RLS as defined by the International RLS Study Group Criteria. Sixty-four percent of FS women had RLS. Those with RLS had more difficulty initiating and maintaining sleep than did FS patients without RLS.
Management and Therapy for Fibromyalgia Syndrome
Since sleep disturbance is a major component of FS, sleep professionals play a role in its management and therapy. Careful evaluation should include screening for primary sleep disorders including sleep-disordered breathing, especially UARS, RLS, PLMD, and circadian rhythm disorders. Review sleep-wake timing, time in bed, napping, light exposure, and the sleep environment. Also, assess for maladaptive sleep behaviors. Review medications, over-the-counter medications, herbal remedies, caffeine, and alcohol use. Further diagnostic workup and treatment should be tailored to the individual patient’s findings.
Management of FS should include treatment of primary sleep disorders if they are present. Patient management is multidisciplinary and should be flexible and multimodal. Therapies include patient education, cognitive behavioral therapy, relaxation techniques, exercise therapy, and pharmacological therapy. A meta-analysis of controlled trials noted that multicomponent therapy has beneficial short-term effects on many FS symptoms (104).
Nonpharmacologic therapy is very important. Patient education has a therapeutic effect on patient fatigue as well as other symptoms (105). Exercise also impacts symptoms. A Cochrane database review examined randomized controlled trials of exercise interventions in patients with FS (106). Aerobic exercise training had positive effects on global well-being, physical function, and possibly pain and tender point perception. Strength training may also benefit patients with FS; however, further studies are needed for assessing the efficacy of muscle strengthening and flexibility (106). Cognitive behavioral interventions are also effective. Multiple randomized controlled trials with longitudinal data of up to 30 months show that these interventions improved function (105). Furthermore, meditation improved depression symptoms in women with FS in a randomized controlled trial (107). Hydrotherapy utilizing spa-type bath therapies improved short-term outcomes in controlled trials (108).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree