Sleep-related Ventilation and Oxygenation
Sleep is associated with a diminished responsiveness of the respiratory center to chemical, mechanical, and cortical inputs. These changes are more pronounced in rapid eye movement (REM) sleep, during which there is more variability in respiratory rate and tidal volume (
1).
Therefore, one can expect the mandatory increase in PaCO
2 and reduction in PaO
2 of 2 to 8 mm Hg and 3 to 10 mm Hg, respectively. Regardless of the change in PaO
2, the SaO
2 declines only by <2% in this scenario, given the characteristics of the oxyhemoglobin dissociation relationship (
2). In individuals with normal pulmonary function and at sea level, there is relatively little consequence to SaO
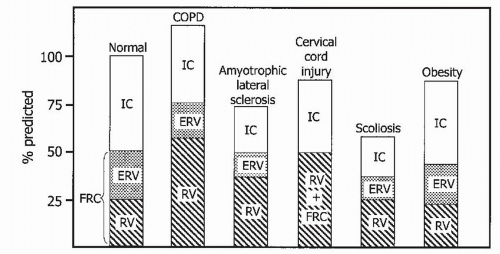
2. The impact of sleep in patients with COPD has been long-studied and, indeed, poses several challenging management considerations. Chronic bronchitis patients—the “blue bloaters”—are more likely to be hypoxemic and hypercapnic as compared to patients with advanced emphysema, the so-called “pink puffers” (
3). Patients with COPD have been noted to demonstrate significant increase in mean pulmonary artery pressures and PaCO
2 levels when going from wakefulness to sleep (
4). The tendency for worsening nocturnal hypoxemia in these patients, coupled with the pulmonary vasculature’s response of hypoxic vasoconstriction, predisposes to the development of pulmonary hypertension and cor pulmonale (
5). These consequences may be partly explained by the effects of the potent pulmonary vasoconstrictor endothelin (ET)-1. Patients with COPD who desaturate at night have higher circulating levels of ET-1 in their sera than do those who do not desaturate (
6).
Nighttime Evaluation
The American Sleep Disorders Association recognizes four different levels of recording devices for the assessment of sleep apnea (
31). Level I includes standard polysomnography (PSG) performed in the laboratory. Level II studies are performed outside of the laboratory and are capable of recording complete PSG without a technologist present (unattended). Level III devices can record respiratory effort, airflow, oxygen saturation, and pulse or ECG. These devices have been the subject of recent guidelines for use as a diagnostic in patients with a high pretest probability of OSA (
32). Level IV devices record only one or two variables such as pulse, oximetry, and ECG.
PSG is the recommended test for the diagnosis of OSA and various disorders of sleep, such as periodic limb movements of sleep (PLMS), upper airway resistance syndrome (UARS), REM behavior disorder, somnambulism, and parasomnias (
31).
Interestingly, the sensitivity of PSG in the diagnosis of OSA has not been clearly shown, as PSG is usually assumed to be the gold standard of comparison. However, there can be substantial night-to-night variability in the apnea—hypopnea index (AHI) on PSG studies. It is also well recognized that a negative overnight PSG does not completely exclude the diagnosis of OSA. This is particularly the case for patients who have risk factors for SDB on clinical grounds. Meyer et al. (
33) studied 11 such patients who met diagnostic criteria for OSA during a second study, with an increase in the AHI from 3.1 ± 1.0 at baseline to 19.8 ± 4.7 events per hour (mean ± SEM,
p < 0.01) on the repeat study. Studies have shown that 11.5% of patients with an AHI of <5 on study night 1 have an increase in the AHI to >5 on study night 2 (
34).
Levels II and III devices refer to portable devices that are useful in situations where symptomatic patients who suggest OSA require prompt evaluation and treatment when PSG is not available. These devices have not been adequately studied in patients with COPD and are not yet recommended for routine clinical use in these patients (
31,
32).
Oximetry, the mainstay of level IV devices, measures the oxygen saturation of hemoglobin by distinguishing deoxyhemoglobin from oxyhemoglobin based on the differential absorption of light (
35). The normal value for oxyhemoglobin saturation during sleep in healthy subjects is approximately 96.5% (±1.5%). There are no decreases reported in oxyhemoglobin saturation for different ethnic populations or sex, but it will decrease with altitude.
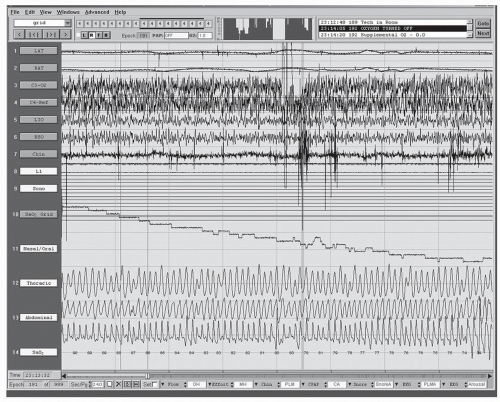
Historically, pulse oximetry was the key means to identify patients with Pickwickian syndrome with prolonged oxygen desaturation (
Figs. 34-2 and
34-3) or severe sleep apnea syndrome with alveolar hypoventilation. The latter may be associated with a saw-toothed pattern (
Fig. 34-4) (
36). Over the last decade, multiple studies have evaluated the utility of pulse oximetry in screening for SDB. The term SDB encompasses OSA, central sleep apnea (CSA) syndrome, UARS, and sleep hypoventilation syndrome. Sensitivities ranging from 31% to 98% and specificities ranging from 41% to 100% have been reported. Williams et al. (
37) used home oximetry and laboratory PSG to evaluate 40 patients suspected of having OSA. Sensitivity of oximetry was only 56%, whereas specificity was 100%. Epstein and Dorlac (
38), retrospectively, reviewed overnight sleep studies on 100 consecutive patients. They used either a “deep” pattern of a ≥4% decrease in SaO
2 to <90% or a “fluctuating pattern” without a definite criteria for change or nadir in the SaO
2 to identify events. Oximetry was considered abnormal when there were 10 or more oximetry events per hour. The fluctuating pattern had a greater sensitivity, whereas the deep pattern had a greater specificity in identifying OSA. If patients with only mild symptoms were considered, the fluctuating pattern was still not as sensitive as PSG.
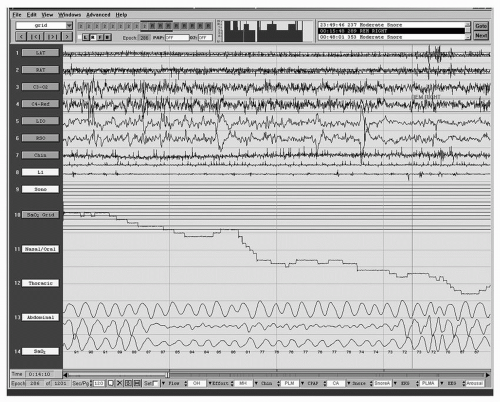
Overnight oximetry can be a useful screening test for SDB when there is a low clinical suspicion of sleep apnea. In a patient able to sleep efficiently for a sufficient time compared with what he or she would consider a routine night of sleep, a normal or negative oximetry can help exclude sleep apnea. Similarly, when there is a high degree of clinical suspicion for OSA and oximetry results are normal, further testing is required. The key characteristics of an abnormal overnight oximetry that suggest SDB are oscillatory variation in saturation and oscillatory desaturation (
Fig. 34-4). Epstein and Dorlac (
38) suggest that this abnormality can be defined as a >4% change in oxyhemoglobin saturation to ≤90% and a pattern of repetitive short-duration fluctuations in saturation and desaturations. Other investigators (
39) have found that resaturations of ≥3% SpO
2 within 10 seconds at the end of a respiratory event were a better detector of respiratory disturbance events (RDEs). Low baseline saturations that may slowly increase or decrease about baseline values without oscillations may be more indicative of gas-exchange abnormalities, such as those encountered in advanced COPD (
Figs. 34-2 and
34-3). The desaturations, secondary to COPD, tend to last much longer and have a much lesser degree of slope in the waveform (
36).
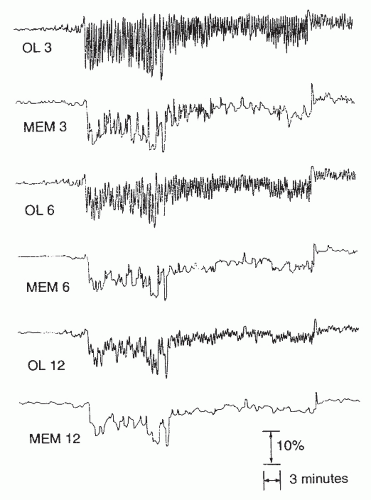
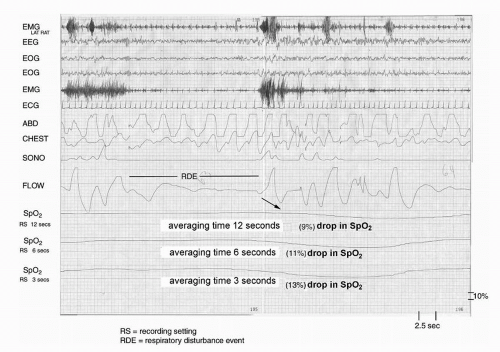
The accuracy of oximetry can be affected by factors such as vasoconstriction, hypotension, skin pigmentation, nail abnormalities, patient movement, and probe positioning. Significantly, different saturation data are obtained at various acquisition options. Davila et al. (
40) studied 75 patients with suspected OSA with simultaneous pulse oxyhemoglobin saturation traces at three recording settings, or averaging times. These investigators found that faster recording settings (shorter averaging times or response times) resulted in lower levels of oxyhemoglobin saturation than did slower settings (longer averaging times or response times) (
Figs. 34-4 and
34-5). Several authors
have also found significant differences in the saturation data obtained online real time (high sampling rates) and those values obtained from memory (low sampling rates) in unattended studies. Desaturation indexes obtained from memory have been found to be significantly lower than those obtained online (
40). Studies have confirmed lower sensitivities and higher specificities using longer averaging times settings (e.g., 12 seconds) and memory display mode (
40). Faster recording settings (shorter averaging times) and online display mode give rise to higher sensitivity and lower specificity. Therefore, the default settings must be known to the user and the interpreter. Faster recording settings and online display are the methodology of choice.
Indeed, oximetry alone is not recommended for the evaluation of sleep apnea in place of PSG, given the lack of standardization in performance and interpretation (
32). Oximetry is, however, comparatively inexpensive, less disruptive to sleep, and more readily available than PSG. It has proved to be very useful in following the response to therapies for OSA, such as oral appliances.
Overnight pulse oximetry is also useful for determining the degree to which patients with COPD desaturate during sleep. Patients with COPD and daytime hypoxemia (PO
2 <55 mm Hg) or PO
2 55 to 59 mm Hg with signs of end-organ damage have improved survival with continuous diurnal and nocturnal oxygen therapy. In the Nocturnal Oxygen Therapy Trial (
41), 203 patients with hypoxemic COPD were randomized to continuous or 12-hour nocturnal supplemental oxygen. One-year follow-up demonstrated improved survival and decreased morbidity with continuous supplemental oxygen. However, there was no improvement in morbidity or mortality with nocturnal oxygen therapy alone. COPD patients with FEV
1 of <1 L may spend >90% of the night with saturations well below 90% when breathing room air (
3,
4). Risk factors for nocturnal desaturation are PaCO
2 ≥45 mm Hg and PaO
2 <65 mm Hg on oxygen (
3,
4). However, the
severity of nocturnal desaturation in patients with COPD cannot be predicted with certainty. Hence, we can see the potential utility of overnight oximetry. The causes of the nocturnal desaturation include hypoventilation, coexisting sleep apnea, reduction in FRC, and altered ventilation-perfusion matching, as previously mentioned. The benefit of nocturnal oxygen therapy in patients with a daytime PO
2 of >60 mm Hg, but nocturnal desaturation, is unproved. However, many clinicians will prescribe oxygen treatment if there is prolonged desaturation or evidence of end-organ dysfunction (cor pulmonale).
Approximately 10% to 15% of patients with COPD have sleep apnea (
1). In COPD patients with signs and symptoms of OSA and/or polycythemia or pulmonary hypertension with right heart dysfunction or right heart failure, a sleep study is indicated. The American Academy of Sleep Medicine outlines other associated features and laboratory findings in many patients with SDB (
42).
Asthma and Other Obstructive Lung Diseases
PSG has demonstrated decreased sleep efficiency, increased arousals and awakenings, decreased sleep time with associated daytime sleepiness, and impaired cognition in patients with asthma (
24,
28). The resulting sleep deprivation may be associated with impaired ventilatory drive and contribute, along with other factors, to the worsening of hypoxemia and hypercapnia in severe acute attacks. In spite of these findings and in the absence of other symptoms of a specific sleep disorder, PSG is not indicated. Overnight oximetry may be used as described for COPD, to evaluate for nocturnal hypoxemia and plan home oxygen therapy.
Patients with CF may have more severe sleep disruption than in other obstructive lung diseases, with impaired daytime functioning. Dancey et al. (
43) reported that 19
patients with severe CF had reduced sleep efficiency (71%) and frequent awakenings, as well as lower mean SaO
2, when compared with 10 healthy controls. Furthermore, patients with CF were sleepier, with reduced sleep latency on multiple sleep latency test (MSLT) (6.7 minutes). These findings correlated with more reported fatigue and lower levels of happiness and activation as well as impaired cognitive function. Sleep-related complaints such as sleep-onset difficulties, sleep-maintenance trouble, and snoring are commonly seen in adults and children with CF. Children and adolescents with CF have decreased sleep efficiency, prolonged REM latency, and decreased REM sleep percentage when compared with controls (
44). Children with stable CF have more frequent nocturnal cough than do children without CF. The cough is more severe with advancing disease and occurs most frequently in the first hour of sleep (
45).
Predicting nocturnal desaturation appears problematic, as spirometric parameters, awake SpO
2, were not found to be predictive in 70 patients with CF studied by Frangolias and Wilcox (
46). However, Milross et al. (
47) reported that evening arterial PaO
2 did contribute to the ability to predict both sleep-related desaturation and elevated transcutaneous CO
2 in 32 stable patients with CF in transitioning from nonrapid eye movement (NREM) sleep to REM sleep. Further studies are needed to determine whether evening ABGs and/or nocturnal oximetry may be useful in the management of these patients with moderate to severe disease (
16,
39,
40 and
41).