Fig. 31.1
Afferent and efferent connections of the medullary respiratory center. (From Fig. 19.4, [13], with permission)
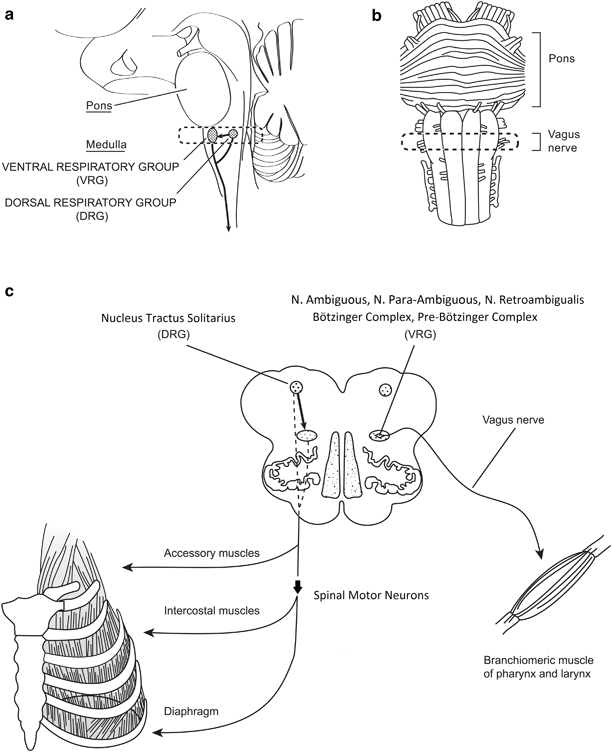
Fig. 31.2
Neuroanatomy of the medullary respiratory center. The brain stem showing in a parasagittal section a the location of the dorsal and ventral respiratory groups (DRG and VRG) and their projection to the spinal cord; in a basal brain view b vagus nerve rootlets exiting the lateral surface of the medulla, and in a transverse section of the medulla c projections from the DRG to VRG, and the output from both groups to respiratory muscles. (From Fig. 19.5, [13], with permission)
Early Studies: Sleep Apnea and Stroke, an Association
Snoring
Prior to the ready availability of polysomnography (PSG), OSA was often suspected on a clinical basis from the major signs and symptoms of what now constitutes the OSA syndrome , as a continuum has been shown to exist between snoring, obesity, and OSA [14, 15].
In 1989, Palomake et al. suggested that snoring alone was a risk factor for stroke [16]. In their study of 167 men with stroke, 36 % suffered stroke in sleep . Stepwise multiple logistic regression analysis found snoring to be the only potential risk factor significantly related to stroke in sleep. In 1991, it was reported that the odds ratio (OR) of snoring increased when the combination of obesity and sleepiness (classical signs/symptoms of OSA) was also present [17]. Retrospective analysis of pioneering studies such as these led to the routine use of PSG in similar research, as a significant number of these subjects were suspected to have had OSA.
Early PSG Studies
The foundation of PSG is electroencephalography (EEG). In 1875, Caton performed the first animal studies and in 1929 Berger published recordings from humans [18–21]. By 1936, there were only six EEG laboratories in the USA, including our facility at the University of Iowa, under the direction of my (MED) mentor John Knott [21]. The first continuously recorded all-night EEG sleep studies by Loomis et al. in 1937 were followed by the discovery of rapid eye movement (REM) sleep by Aserinsky and Kleitman in 1953, which showed the utility of the electrooculogram (EOG) [22, 23]. In 1967, Jouvet showed the association of REM sleep with hypotonia justifying the present use of the electromyogram (EMG) [24]. In 1968, the combination of EEG, EOG, and EMG allowed for formal PSG and the first published standardized technique for scoring sleep stages by Rechtschaffen and Kales [25]. In 1978, utilizing the PSG, Guilleminault et al. defined the term apnea index (the average number of apneas and hypopneas per hour of sleep), to precisely define the presence and severity of OSA [26]. In 2007, the American Academy of Sleep Medicine published the first standard definition for hypopnea in the pediatric population, thus allowing for the routine reporting of the apnea–hypopnea index (AHI; the average number of apneas plus hypopneas per hour of sleep) in children [27]. Today, the AHI is “…the key measure used for case identification, for quantifying disease severity, and for defining disease prevalence in normal and clinical populations” [28] (Table 31.1).
Table 31.1
Early reports of stroke patients/populations studied with PSG. (From [68], with permission)
Study type | Number of subjects | % OSAa | AHIb/RDIc | SaO2 (%)d | Location | Stroke type (number of patients) |
|---|---|---|---|---|---|---|
Stroke case reports | – | – | – | – | – | – |
Chaudhary et al. [29] | Stroke = 1 | 100.0 | 18.0 | 60.0 | Brainstem | I |
Tilkare et al. [30] | Stroke = 1 | 100.0 | 78.0 | 70.0 | Hemispheric | I |
Rivest/Reiher [41] | Stroke = 1 | 100.0 | NG | NG | Brainstem | I |
Askenasy/Goldhammer [100] | Stroke = 1 | 100.0 | 25.0 | 80.0 | Brainstem | I |
Dyken et al. [31] | Stroke = 1 | 100.0 | 36.0 | 60.0 | Subcortical | H |
Pressman et al. [42] | Stroke = 1 | 100.0 | 22.0 | < 50.0 | Hemispheric | I |
Selected stroke populations | – | – | – | – | – | – |
Kapen et al. [36] | Stroke = 47e(31) | 72.0 | 28.0 | NG | Hemispheric | I |
Good et al. [101] | Stroke = 19 | 95.0 | 36.0 | NG | Hemispheric (16) Brainstem (3) | I |
Case–control studies | – | – | – | – | – | – |
Dyken et al. [9] | Stroke = 20 | 70.0 | NG | NG | NG | NG |
Hudgel et al. [37] | Stroke = 8 Control = 8 | NG NG | 44.0 12.0 | 82.0 90.0 | NG NA | I/H NA |
Mohsenin/Valor, [38] | Stroke = 10 Control = 10 | 70.0 (OSA) 10.0 (CSA) 0 | 52.0 3.0 | 70 % 80–84 % | Hemispheric (7) Subcortical (1) | I (9) H (1) |
Dyken et al. [10] | Stroke = 24 Control = 27 | 71.0 19.0 | 26.0 4.0 | 85.0 91.0 | Hemispheric (12) Subcortical (8) Cerebellar (2) Brainstem (2) | I (20) H (4) |
Bassetti et al.f [43] | Stroke = 23 Control = 19 | 70.0 16.0 | 32.0 6.0 | 82.0 89.0 | Anterior circ. (74 %) Posterior circ. (26 %) | I |
Bassetti et al. [102] | Stroke = 39 | 54.0 (OSA) 10.0 (CSB) | 26.0 (NREM) 30.0 (REM) | 82.0 (ST) 83.0 (IT) | Hemispheric (28) Brainstem (9) Pontocerebellar (1) Cerebellum (1) | I |
Stroke Case Reports and Case Series
In 1982, Chaudhary et al. published a PSG study of a 46-year-old hypertensive man (performed after a right lateral medullary infarction) that showed an apnea index of 18, with an oxygen saturation (SaO2) low of 60 % [29]. Injury to the nucleus ambiguous was hypothesized to have caused the OSA. Nevertheless, reports of progressive weight gain, snoring, and sleepiness suggested OSA was present prior to stroke .
In 1985, Tikare et al. studied a subject with PSG-documented OSA and recent stroke (the brain computerized tomogram (CT) revealed paraventricular areas of low attenuation) [30]. To our knowledge, this chapter was the first to suggest that OSA-induced hypoxia and cardiac arrhythmia might cause stroke.
In 1991, our group’s research interest was piqued by a sleepy, obese 34-year-old man, with a history of snoring, who awoke with left hemiparesis, after which PSG diagnosed severe OSA [31]. A brain CT suggested he had suffered a hypertensive hemorrhagic stroke (Fig. 31.3). Our hypothesis, that this stroke could have represented an OSA-precipitated hypertensive bleed, was supported by previous hemodynamic studies which reported elevated blood pressures frequently followed sleep-related obstructive respirations [32–35].
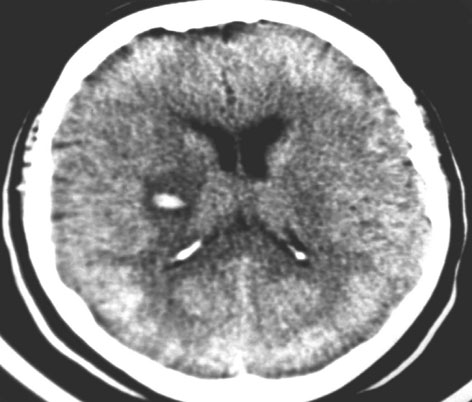

Fig. 31.3
This brain CT without contrast was performed in a 34-year-old man who awoke with stroke, after which he was diagnosed with obstructive sleep apneas. This study reveals a hemorrhage, with a surrounding area, greater than 1 cm in diameter, of low density, consistent with edema, involving the putamen and posterior limb of the right internal capsule. (From [68], with permission). CT computerized tomogram
In 1991, Kapen et al. selected 31 subjects with ischemic stroke for PSG [36]. They combined the findings of these studies with those of 16 similar patients who had incomplete sleep studies without respiratory effort or SaO2 monitoring (S. Kapen, MD, personal communication, 1997). OSA was found in 72 % of these subjects.
Case–Control Stroke Studies
In 1992, our group presented preliminary data from a 4-year follow-up study of 24 consecutively encountered inpatients admitted for stroke, and 27 healthy gender- and age-matched control subjects without stroke [9, 10]. OSA (defined as either an AHI ≥ 10, or any number of obstructive events associated with an SaO2 value < 86 %) was found in 71 % of strokes. Closer analysis showed OSA in 10 of the 13 males with stroke (77 %) and in only 3 of the 13 males without stroke (23 %) (P=0.0169). Seven of the 11 females with stroke (64 %) had OSA, whereas only 2 of the 14 females without stroke (14 %) had OSA (P = 0.0168).
In 1993, Hudgel et al. selected eight elderly patients, with finger pulse oximetry studies suggesting apnea, and stroke histories ≥ 1 month, for PSG [37]. Stroke subjects were matched to controls in regard to age, gender, weight, and height. Although the type of apnea and the number of individuals with sleep apnea were not defined for either subject group, the respective mean AHI for strokes and controls was 44.0 and 12.0. The authors suggested that apnea-induced hypoxemia and oscillations of intracranial pressure and blood circulation might increase the risk for ischemic stroke .
In 1995, Mohsenin and Valor selected ten patients from a rehabilitation facility with < 1-year histories of hemispheric stroke, for PSG analysis [38]. None had prior apnea, snoring, sleepiness, obesity, or neurological problems. A control group was matched for age, obesity, smoking, and hypertension . The respective mean AHIs for the stroke and control groups was 52.0 and 3.0. The authors hoped to provide new evidence for a cause-and-effect relationship between hemispheric stroke and sleep apnea. Nevertheless, bias was placed against OSA causing stroke as subjects with histories of snoring, obesity, and apnea were selected out.
OSA: A Stroke Risk Factor
Transient Ischemic Attacks
Transient ischemic attacks (TIAs; “ministrokes”) are focal neurological deficits resolving within 24 h [3]. Case reports of patients with histories of TIAs during sleep, obesity, and snoring, after the treatment of their PSG-documented OSA led to no further recurrence of TIA, indirectly suggest a cause-and-effect relationship between OSA and stroke, as 15 % of strokes are preceded by TIA (90-day risk; up to 17.3 %) [3, 39–42]. Nevertheless, case-controlled study reports have been inconsistent and OSA is not considered a TIA risk factor [43, 44].
Cohort Studies
Large population and clinically based cohort studies, despite using variable populations, PSG methodologies, OSA definitions, and statistical analyses, and often examining stroke risk in combination with other medical problems, suggest that adults with OSA and an AHI ≥ 20 are at stroke risks (Table 31.2).
Table 31.2
OSA and stroke risk: incidence studies. (Modified from Table 1 [8], with permission)
Incidence studies | Subjects studied | Population size | AHI used to define SDB group | SDB prevalence | AHI used to define comparison group | Mean follow-up period (years) | Group outcome Total number of subjects in a given group with outcome | Risk estimate of SDB as risk factor for outcome (95 % CI) |
|---|---|---|---|---|---|---|---|---|
Authors/year | ||||||||
Arzt et al. [46] | Population based General adult 30 60 years | a1475 b1189 | ≥ 20 | 7 % ( N = 99) | < 5 | Three intervals of 4 years | Stroke SDB = 4 Comparison = 9 | cOR = 4.48 (1.31–15.33; P = 0.02) |
Yaggi et al. [47] | Clinic based Referred for suspected SDB ≥ 50 years | 1022 | ≥ 5 | 68 % ( N = 697) | < 5 | 3.4;SDB 3.3;CG | Stroke, TIA, or death SDB = 72 Comparison = 16 | dHR = 1.97 (1.12–3.48; P = 0.01) |
Munoz et al. [48] | Clinic based: Random sample, noninstitutional elderly 70–100 years | 394 | ≥ 30 | 25 % ( N = 98) | < 30 | 4.5 | TIA or ischemic stroke SDB = 9 Comparison = 11 | eHR = 2.52 (1.04–6.1; P = 0.04) |
Valham et al. [49] | Clinic based Symptomatic angina and CAD | 392 | ≥ 5 | 54 % | < 5 | 10.0 | Stroke SDB = 38 Comparison = 9 | fHR = 2.89 (1.37–6.09; P = 0.005) |
Redline et al. [50] | Community based ≥ 40 years | 5422 | ≥ 15 | Male 44 % ( N = 1095) Female 24 % ( N = 720) | < 4.1 | 8.7 | Ischemic stroke Male SDB = 54 Female SDB = 37 | Male: gHR = 2.86 (1.1–7.4) Female: hA 2 % increase in HR (0–5) after threshold OAHI of 25 |
Population-Based Studies
In 2001, Shahar et al. examined the cross-sectional association between sleep-disordered breathing (SDB) and self-reported CVD in 6424 subjects using home (unattended) PSG [45]. Mild-to-moderate SDB was highly prevalent with a median AHI of 4.4; inter-quartile range, 1.3–11.0. At least one element of CVD (stroke, angina, myocardial infarction , heart failure , or coronary revascularization procedure) was reported by 16 % ( N = 1023) of subjects. The relative odds ratio (95 % confidence interval (CI)) of prevalent stroke (upper vs. lower AHI quartile) was 1.58 (1.02–22.46). This data suggested even mildly elevated AHI values have modest-to-moderate effects on CVD.
In 2005, Arzt et al. addressing 12 years of data from a stratified random sample performed a cross-sectional analysis, utilizing logistic regression, of 1475 subjects (30–60 years) [46]. A baseline AHI ≥ 20 independently increased the OR for stroke (3.83; 95 % CI, 1.17–12.56; P = 0.03) compared to a reference group with an AHI < 5, after adjusting for confounding factors (age, sex, body mass index (BMI; weight in kilograms divided by square of height in meters), alcohol, smoking, diabetes, and hypertension) .
From the original population, a longitudinal analysis of 1189 individuals tested whether SDB was associated with increased incident stroke at 4-year intervals throughout the study (at 4, 8, and 12 years). A baseline AHI ≥ 20 had significantly higher OR for incident stroke compared to the reference group (4.48; 95 % CI, 1.31–15.33; P = 0.02), using a model that controlled for age and sex.
Clinic-Based Studies
In 2005, Yaggi et al. studied an observational cohort of 1022 subjects with suspected SDB [47]. They compared the combined risk of developing composite stroke, TIA, or death from any cause in 697 subjects with OSA (AHI ≥ 5), to individuals with an AHI < 5. Many with SDB received treatment during the study, including diet, positive airway pressure (PAP) therapy, and upper airway surgery.
Follow-up over 3.3–3.4 years of 842 total study subjects showed 22 incident strokes and/or TIAs and 50 deaths in the OSA group, whereas there were only two strokes and/or TIAs and 14 deaths in the comparison group. After adjusting for age, sex, BMI, diabetes, hyperlipidemia, atrial fibrillation, hypertension , race, and smoking, OSA was associated with a significant risk for composite stroke, TIA, or death (hazard ratio (HR), 1.97; 95 % CI, 1.12–3.48; P = 0.01). Trend analysis found an increase in OSA severity associated with increased risk of stroke, TIA, or death from any cause ( P = 0.005).
In 2006, Munoz et al. studied a noninstitutionalized elderly population (range: 70–100 years; median: 77.28), drawn from a random one-stage cluster sampling stratified by age, sex, and census area [48]. After adjusting for sex, a baseline AHI ≥ 30 was a risk factor for incident ischemic stroke or TIA, with a HR of 2.52 (95 % CI = 1.04–6.1, P = 0.04).
In 2008, Valham et al. studied a population < 70-years old, with symptomatic angina and coronary artery disease (verified by angiography and left ventriculography) [49]. The first evaluations of 392 patients randomly selected for modified PSG, without electroencephalography (EEG), using a pressure sensitive bed to monitor respiration, showed sleep apneas (AHI ≥ 5) in 54 %. All patients were then followed for 10 years (nine receiving OSA therapies), during which 47 (12 %) had strokes. Increased stroke risk was associated with an initial diagnosis of obstructive sleep apneas; HR of 2.89 (95 % CI 1.37–6.09, P =0 .005), independent of OSA treatment, previous stroke or TIA, sex, age, BMI, diabetes, hypertension , atrial fibrillation, left ventricular function, or smoking. Independent of confounders, an AHI > 5 and < 15, and an AHI ≥ 15, respectively, had 2.44 (59 % CI 1.08–5.52, P = 0.011) and 3.56 (95 % CI 1.56–8.16, P = 0.011) times increased risk of stroke compared to those without apnea.
In 2010, Redline et al. followed 5422 subjects, without a history of stroke, for a median of 8.7 years, during which 193 new ischemic strokes occurred [50]. A significantly positive association between initially documented obstructive apnea/hypopnea indices (OAHI) and new ischemic stroke was seen. The greatest risk was for men with an OAHI > 19.1 (the top quartile), with an adjusted HR of 2.86 (95 % CI 1.1, 7.4), when compared to men with an OAHI < 4.1 (quartile I). In women, stroke risk was not associated with OAHI quartile or oxygen desaturation. Nevertheless, there was, using nonlinear, covariate-adjusted associations with the OSA exposures and interactions with gender, a 2 % increase (95 % C.I. 0, 5) in stroke HR with each unit increment in OAHI after a threshold of 25.
Treatment Versus Non-treatment
Morbidity and Mortality
In 1996, we published a 4-year follow-up on all stroke and sex- and age-matched control subjects from a 1992 prevalence study of OSA in stroke, with the exception of three controls who moved, leaving no contact information [9, 10]. All stroke subjects who died had OSA ( N = 5), of whom only one used continuous PAP (CPAP; death from urosepsis). Only one male control died; without OSA, from prostatic carcinoma. Respectively, stroke subjects dead versus alive had mean AHIs of 41.3 and 22.1, suggesting the diagnosis and severity of OSA in stroke were associated with greater long-term mortality.
A 1990, prospective, treatment versus non-treatment study by Partinen et al. has been used to suggest a cause-and-effect relationship between OSA and stroke [51, 52]. Over 7 years, they followed 198 OSA subjects treated with either weight loss ( N = 127) or tracheostomy ( N = 71); 5.2 % of the weight loss group had stroke (17.3 % died, 11 % from vascular etiologies), whereas only 1.2 % with tracheostomy suffered stroke (2.8 % died).
CPAP
In 2005, Martinez-Garcia et al. showed, of 51 patients with recent stroke and minimal AHI of 20, only 15 (29.4 %) tolerated CPAP after 1 month [53]. In those who could not tolerate CPAP, the probability of a new vascular event increased fivefold (OR 5.09) .
In 2006, Palombini et al. studied 32 patients with recent stroke [54]. Only 22 % tolerated CPAP for 8 weeks; difficulties with CPAP use as perceived by the patient and family members, facial weakness, motor impairment, and increased difficulties and discomfort using a full-face mask. Brown et al. found stroke patients took longer to put on ( P< 0.01) and remove ( P< 0.01) traditional headgear as compared to a one-piece system [55]. Palombini et al. stated, “Better education and support of patients and families, and special training session in rehabilitation services, will be needed to improve compliance” [54]. Disler et al. showed, in a supportive setting, patients with stroke and OSA, despite moderately severe motor and cognitive functional independence measurement scores, tolerated CPAP with normalization of oxygen saturations [56].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





