- Obstructive sleep apnoea (OSA) syndrome most commonly develops on the background of severe snoring and affects around 4% of middle-aged males
- Most, but not all, patients with OSA have significant central obesity affecting abdominal and neck girth
- The most obvious consequence of OSA syndrome is unrefreshing overnight sleep with significant subsequent daytime somnolence, predisposing to accidents and poor work performance
- Increasingly, the adverse metabolic and cardiovascular effects of OSA on general health are recognised as potentially reversible phenomena
- Continuous positive airways pressure (CPAP) therapy is the best proven treatment for OSA although alternative approaches are available if it is unsuccessful or inappropriate
- Mandibular advancement devices may be successful in mild or moderate OSA
- Central sleep apnoea (CSA) occurs when subjects fail to initiate breathing when asleep and has a variety of causes and associations, most notably significant heart failure
- Treatment of CSA is complex and may not always be necessary if there are no adverse daytime consequences
It is perhaps unfortunate that snoring is often perceived as something of a laughing or trivial matter. However, very severe snorers may obstruct their airways so frequently as to partially arouse them from sleep over 500 times a night, thereby ruining sleep quality and potentially causing life-threatening daytime drowsiness. The secondary adverse effects of sleep-disordered breathing on daytime performance, cognition, quality of life, mood and general medical health, as well as the sleep efficiency of bed partners, are increasingly recognised.
At least a third of middle-aged males snore habitually, with around 10% producing noise levels potentially audible in adjacent rooms. Although prevalence figures vary across populations, up to 3% of males and 2% of females over 50 may have obstructive sleep apnoea syndrome (OSAS), an important and eminently treatable condition.
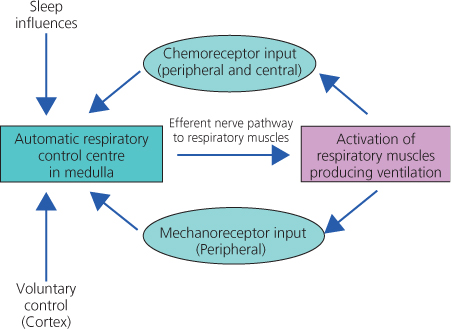
During sleep, the control of breathing is involuntary, regulated by an automatic negative feedback system, largely centred in the lower brainstem (Figure 4.1). The medullary control centre receives information about acidity and gas concentrations from chemoreceptors situated both in the brain itself and in carotid arteries. A number of mechanoreceptors from the respiratory tract and muscle spindles in respiratory muscles also influence the system.
In general, breathing in sleep is shallower and the normal homeostatic set points, such as carbon dioxide levels, are less tightly controlled. This occurs particularly during the REM sleep stage, in which respiratory rates become variable.
In normal breathing, air is sucked into the lungs by creating a negative pressure in the upper airways during inspiration. The tendency for the airway to close off is counteracted by persistent or tonic stiffness in muscles, such as the genioglossus and surrounding palate. As sleep deepens, these areas naturally become floppier causing the airway to narrow, potentially producing air turbulence (heard as snoring) or frank obstruction of breathing. A useful analogy refers to the difficulty encountered when drinking through an old-fashioned paper straw. If the straw becomes wet, it collapses on itself, initially causing ‘slurping’ before complete obstruction. Further attempts to suck harder are counterproductive.
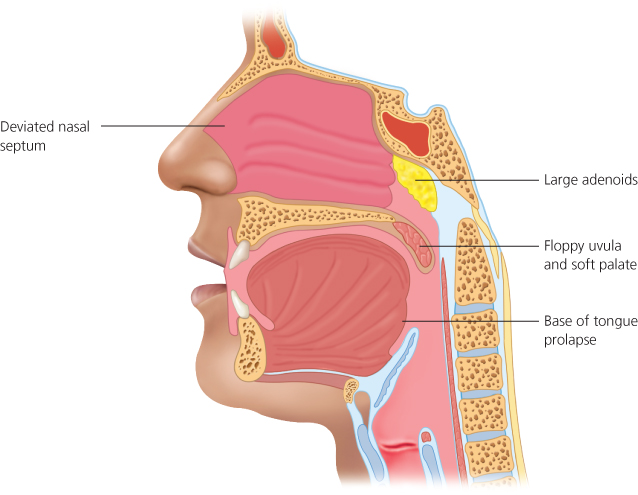
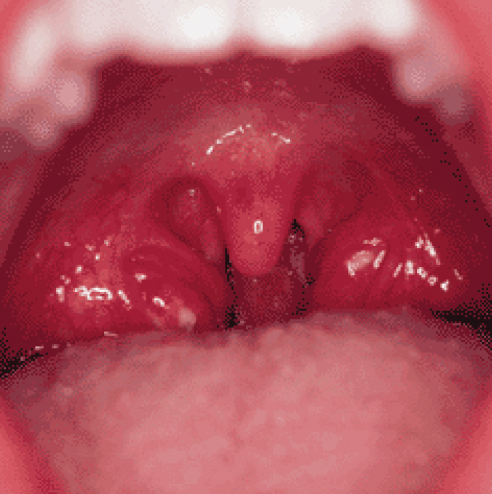
Anatomically determining precisely where the airway obstructs in a snoring subject can be difficult unless there are clear visible physical factors, such as enlarged nasal polyps, adenoids or tonsils (Figures 4.2). The latter are particularly common in children between two and six years of age (Figure 4.3).
An apnoea is arbitrarily defined as cessation of breathing for at least 10 seconds and will quickly lead to hypoxia and a rise in plasma carbon dioxide levels (hypercapnia). If due to obstruction, the detection of these physiological changes automatically leads to increased respiratory effort, sympathetic activation and partial arousal from sleep which may not be registered or recalled consciously by the subject.
In a small proportion of subjects with sleep apnoea, the problem arises because there is reduced or an absent effort to breathe, so-called central sleep apnoea (CSA).
Hypopneas occur when there is partial occlusion enough to reduce airflow by 30% and oxyhaemoglobin levels by 4%, again over a 10-second interval. The apnoea-hypopnea index (AHI) refers to the number of events per hour and is a commonly used measure describing the severity of sleep-disordered breathing. An AHI of 10–20 roughly corresponds to mild sleep apnoea whereas a figure over 50 generally produces severe symptoms. However, OSAS remains a clinical diagnosis and an apparently high AHI may not be clinically significant unless there are adverse daytime consequences, particularly on wakefulness and performance.
Diagnosing obstructive sleep apnoea
Obstructive sleep apnoea causes a variety of symptoms and can, therefore, present to clinicians in a number of ways (Table 4.1). Given its high and increasing prevalence, there should always be a low threshold for considering it. A number of nocturnal neurological problems, including cluster headaches and epilepsy, are associated with OSA and will often improve with successful treatment of the breathing disorder.
Table 4.1 Various symptoms commonly associated with obstructive sleep apnoea.
| Waking symptoms | Nocturnal symptoms |
| Excessive sleepiness or fatigue | Snoring |
| Morning headache or ‘fullness’ | Choking or witnessed apnoeas |
| Poor concentration and attention | Restless sleep |
| Cognitive dysfunction | Sleep maintenance insomnia |
| Personality change | Nocturia |
| Poor work performance | Sweating |
| Erectile dysfunction, reduced libido | Dry mouth through night |
| Sore throat | Acid reflux |
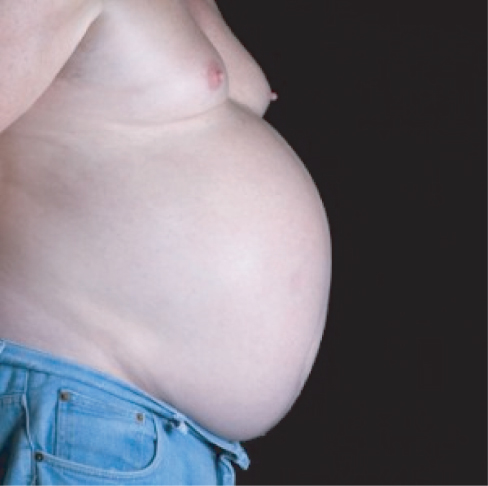
OSA predominantly affects males who have central obesity (Figure 4.4), typically with collar sizes above 17 inches. However, many other factors may predispose an individual to OSA (Table 4.2). In females, for unclear reasons, equivalent levels of OSA may carry a worse prognosis compared to male subjects.
Table 4.2 Factors predisposing to obstructive sleep apnoea.
| Factor | Cause | Comment |
| Small upper airway | Obesity | Particularly central obesity in those who are ‘apple-shaped’; increased neck collar size is clearest risk factor |
| Tobacco smoking | Possibly inflamed upper airways | |
| Hormonal factors | Hypothyroidism and acromegaly may increase soft tissue around airway; post-menopausal women more at risk | |
| Supine position | Snoring generally worse when sleeping on back; a pillow wedged between the shoulders may prevent sleeping in a supine position | |
| Upper airway lesions | Rhinitis, nasal polyps, enlarged tonsils/adenoids may produce OSA in non-obese subjects | |
| Retrognathia or facial deformity | A severely receding chin, either as a normal variant or as part of a developmental syndrome, may increase risk | |
| Reduced dilator activity of upper airways muscles | Sedative drugs or anaesthetics | Alcohol and benzodiazepines are common examples |
| Neurological disorders | Any cause of bulbar muscle weakness; significant strokes commonly produce OSA in early stages of recovery | |
| Increased chest wall resistance | Obesity | Severe truncal obesity or increased muscle mass in bodybuilders may simply ‘weigh down’ the chest during sleep |
OSA may also be overlooked in those who sleep alone and a snoring history is absent. OSA should therefore always be considered in anyone who feels significantly unrefreshed after an apparently adequate period of nocturnal sleep.
Aside from unrefreshing overnight sleep and daytime somnolence, OSA has been linked as an independent contributor to a variety of metabolic and cardiovascular long-term problems (Table 4.3). Many of these presumably occur because the sympathetic arousal systems are persistently activated overnight, although a number may simply reflect the adverse consequences of chronic severe sleep deprivation. The latter may even lead to an impression of significant cognitive decline or even dementia.
Table 4.3 Proposed clinical adverse consequences of OSA.
| Possible adverse consequence | Comment |
| Hypertension | A link with hypertension has been clearly established, particularly overnight when blood pressure normally falls significantly |
| Increased diabetic risk | Increased insulin resistance in OSA has been proven although the precise mechanism remains obscure |
| Obesity | Increased resistance to chemical satiety signals in the brain (notably leptin) has been proposed |
| Hormonal changes | Associated endocrine abnormalities often seen although causation not always established; reduced growth hormone secretion in children with severe OSA is probably a significant clinical consequences |
| Impaired inflammatory response | The sleep deprivation associated with chronic OSA may lead to less effective vaccinations and increased susceptibility to infections |
| Increased cancer risk | A theoretical risk, possibly related to chronic sleep deprivation and reduced immune surveillance |
| Increased brain atrophy | Several large studies have shown reduced grey matter throughout most of the cortex and in part of the cerebellum of severe OSA patients; the mechanism is not established |
The commonest method for diagnosing OSA is through simple home oximetry using a finger probe to detect plasma oxygen desaturations and pulse rate through the night (Chapter 2). A typical positive recording is shown with pulse rate rises accompanying the dips in oxygen levels (Figure 2.3). This is a relatively inexpensive procedure and is useful for monitoring treatment effects.
With oximetry, false positive results may be obtained if oxygen dips are secondary to central sleep apnoea (CSA) and not simple obstruction of the airway. Furthermore, chronic respiratory disease producing chronic hypoxaemia may complicate interpretation. The procedure is also prone to artefacts and will not demonstrate the poor sleep quality of those heavy snorers who do not fully obstruct their breathing but are partially awoken by the increased breathing effort. Such a concept of an ‘upper airways resistance syndrome’ (UARS) is controversial but almost certainly is valid for some subjects.
Depending on resources, suspected cases of OSA in whom oximetry has not been helpful or fully diagnostic may proceed to further overnight investigations. Ambulatory devices are increasingly available and allow recordings at home of several useful parameters. These include oxygen saturations, pulse rate, nasal airflow, respiratory effort deduced from pressure transducers on chest and abdominal surfaces, and leg movements. The last measure may indicate excessive and regular limb restlessness as an additional cause for poor sleep quality.
Full polysomography in a sleep laboratory is sometimes justified to quantify and stage overnight sleep accurately in order to assess the presence and potential contribution of additional sleep disorders.
Treating obstructive sleep apnoea
The aims of treating OSA are to improve overnight sleep quality and daytime sleepiness, reduce the risk of accidents and minimise the associated medical complications such as hypertension.
Initial approaches in general practice
- Obese patients should be encouraged strongly to lose weight, especially if recent weight gain has coincided with an increase in shirt collar size. Arguably, there should be a lower threshold for recommending anti-obesity drugs or bariatric surgery to aid weight loss in the presence of significant OSA. A 10% loss in weight might be expected to reduce the AHI by 25% in an average subject.
- Advice should also be given regarding smoking cessation and excessive alcohol intake.
- Sedating drugs, especially benzodiazepines, should be withdrawn if possible.
- Nasal dilators may improve snoring but rarely improve frank OSA. Inhaled steroids are recommended if there is evidence for rhinitis or nasal polyps.
- It is difficult to alter sleeping posture but physical attempts to avoid rolling over into a supine position are sometimes successful.
Nasal continuous positive airway pressure (CPAP)
In those with established OSAS on the basis of overnight investigations and daytime symptoms, nasal CPAP is now regarded as first-line treatment assuming conservative measures have failed. Nasal CPAP delivers air at increased pressure through inspiration and expiration, acting as a pneumatic splint, gently forcing the airway more open.
A variety of pumps and nasal or full face silicon masks are now available to suit different head shapes and individual preferences. The simplest system uses a fixed pressure of air (typically 6–20 cm H2O) delivered through the night. Some systems are programmed to increase the pressure over the first 30 minutes of sleep if sleep onset is rendered difficult. Other machines have a variable pressure setting and can be used to auto-titrate the correct pressure, aiming to eliminate over 90% of obstructive episodes. A variable pressure or auto-set system is usually better tolerated but more expensive.
The majority of CPAP machines have an internal clock to measure average usage overnight. Unfortunately, long-term compliance is rarely above 50%. Reasons for discontinuation are variable (Table 4.4) and are inversely proportional to the level of education and support available to the patients. In general, the more severe the level of OSA, the more likely the subject is to obtain symptom relief and, therefore, persist with therapy.
Table 4.4 Reasons for discontinuing CPAP therapy.
| Complication of CPAP | Corrective measure |
| therapy potentially | |
| leading to discontinuation | |
| Air leak | Change mask or headgear; try chin strap; consider full face mask |
| Nasal symptoms | Heated water humidification; minimise mouth leak; consider inhaled steroids |
| Skin ulceration | Change mask type; protect skin |
| Claustrophobia | Use smaller mask or nasal seals |
| Aerophagy | Reduce pressure or usage of CPAP |
| Noise of CPAP | Reassure; ear plugs |
| Lack of efficacy on daytime symptoms | Check pressures and repeat oximetry; diagnosis may be incorrect |
Mechanical devices to keep airway open
Mandibular advancement or positioning devices (MADs)
These hold the mandible and hyoid forwards and can be effective in mild or moderate OSA, especially if the obstruction is mainly at the base of the tongue. They advance the mandible to about 75% of its maximum protruding position, at least 50 mm forward. The devices are constructed either of one piece, similar to a gum shield, or of two pieces that are adjustable and usually require fitting by a dental surgeon. The latter are harder, less bulky and generally better tolerated.
MADs cannot be used in subjects who are edentulous or who have had extensive dental procedures. They are less effective in severe obesity or in those with temporo-mandibular joint dysfunction.
Other devices
Nasal dilators can be internal or external and may help with snoring. They rarely improve established OSA, however.
Palatal inserts to stiffen the uvula and palate have been developed. Evidence for efficacy has yet to be established.
Devices to keep the tongue anchored by suction to the anterior part of the mouth tend not to be tolerated. Excessive salivation and soreness usually limit usefulness.
Palatal and tongue surgery
Although a single surgical procedure to widen the airway may seem a relatively simple and attractive approach, it has become increasingly unpopular as a treatment option. With the exception of adeno-tonsillectomy, usually in children, long-term results of surgery are extremely variable, partly because it is often difficult to accurately determine the site of obstruction. Furthermore, the notion that resulting scar tissue will stiffen the palate and help reduce compliance has not been substantiated.
Either uvulo-palatoplasty or tongue resection can be achieved by both conventional surgery and laser or radiofrequency techniques. However, these procedures are rarely successful in severe OSA and are associated with considerable post-operative morbidity. If CPAP therapy is used subsequent to such surgery, mouth leaks are common and necessitate wearing a full face mask.
Particularly in children with lower jaw or facial deformity, orthognathic surgery to expand the maxilla and advance the mandible may be appropriate. This approach is highly specialised and requires detailed pre-operative assessment with imaging.
Wake-promoting drugs
Approximately 5% of subjects with significant OSA fail to respond to best available or first-line treatments such as CPAP. Reasonable controlled evidence suggests that wake-promoting drugs, such as modafinil in standard doses, may be effective supplemental symptomatic treatment. Concerns over side effects such as hypertension and relatively high drug costs have limited this approach, however.
Central sleep apnoea
Although OSA is by far the commonest reason for intermittent cessation of overnight breathing, in some subjects the neural drive or effort to breathe appears impaired. This can be picked up as an incidental finding on an overnight recording with no obvious clinical consequences. In a minority, however, so-called central sleep apnoea (CSA) produces clinically significant hypoventilation overnight with sleep fragmentation and daytime consequences.
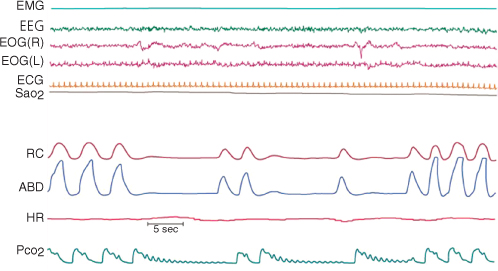
There are four typical scenarios in which nocturnal hypoventilation may be seen (Figure 4.5):
Figure 4.5 In this example of a polysomnogram, the periodic lack of signal in the ribcage (RC) and abdominal (ABD) traces indicates a lack of respiratory effort as is seen in central sleep apnoea. In this recording a sensitive carbon dioxide (CO2) sensor has picked up oscillations in the CO2 partial pressures (pCO2) during the apnoeic periods. This reflects small displacements of air at the nostril caused by a beating heart, proving the patency of the upper airways.
ECG, electrocardiogram; EMG, electromyogram; EOG, electro-oculogram; HR, heart rate; SaO2, oxygen saturation in blood