Sleep Disorders: Introduction
This chapter primarily focuses on sleep and sleep disorders in adults. While many basic and clinical aspects are similar in children, developmental issues and some disorders not present in adults, for example, sudden infant death syndrome, are beyond the scope of this chapter. For further information the reader is referred to Principles and Practice of Sleep Medicine in the Child (Ferber and Kryger, 1995).
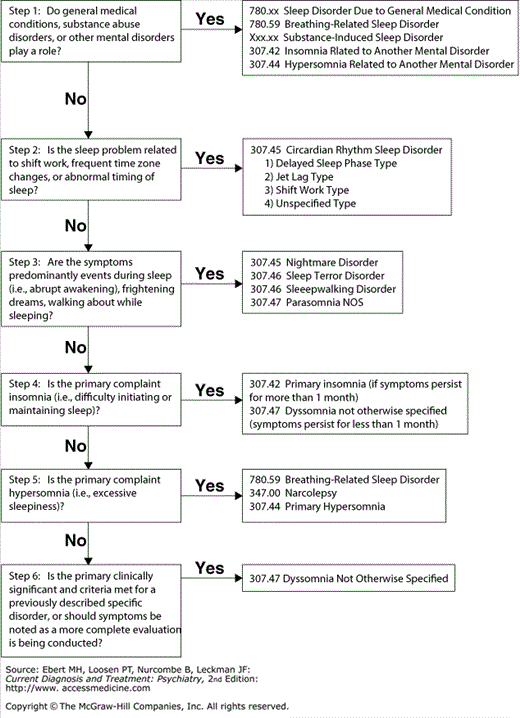
Clinicians should ask routinely about sleep and wakefulness. A thorough sleep history lays the foundation for accurate diagnosis and effective treatment of sleep disorders (Table 27–1). Patients’ sleep complaints will usually fall into four general categories: Complaints of difficulty initiating sleep or staying asleep (insomnia), difficulty staying awake during the day (hypersomnia); abnormal movements or behavior during sleep (parasomnia), timing of the sleep–wake cycle at undesired or inappropriate times over a 24-hour day (circadian rhythm disorder), or a combination of the above (see Figure 27–1).
|
During the evaluation, the patient’s bed partner or other informants should be included whenever possible. Since the patient may be unaware of sleep and wakefulness difficulties, bed partners often initiate the sleep evaluation for sleep apnea, periodic limb movements (PLMs) during sleep, or excessive daytime sleepiness (EDS). Sleep disorders can be disruptive to a household, not just the patient (e.g., sleepwalking, loud snoring).
The clinician should take a thorough history of all pertinent medical and psychiatric problems and family history, review medications, personal relationships, environmental stressors, and review of systems and complete a physical examination including a thorough neurologic examination.
Most sleep complaints can be managed by the nonspecialist, with the motivation and cooperation of the patient, through behavioral modification, treatment of underlying and comorbid diagnoses, and appropriate use of medication for symptomatic relief of sleep-related symptoms. For sleep apnea, PLMs during sleep, narcolepsy, parasomnias with potential for serious injury, or intractable insomnia, referral to a sleep disorder center should be considered.
Nocturnal polysomnography (PSG) records the patient’s sleep overnight in a sleep laboratory. Polygraphic sleep recordings are obtained in a quiet, dark, comfortable laboratory environment. Surface electrodes are affixed to the skin to monitor the electroencephalogram (EEG), bilateral eye movement activity or electrooculogram (EOG), and chin muscle tonus or electromyogram (EMG). Sleep staging is determined by a scanning of these tracings visually. The anterior tibial EMG reflects PLMS when present. Additional physiologic monitoring includes respiratory effort monitoring of the chest and abdomen, airflow such as end tidal CO2 or nasal-oral thermistor, blood O2 saturation, and electrocardiogram.
Changes in EEG frequencies discriminate waking and nonrapid eye movement (non-REM) sleep stages; the concurrent presence of eye movements in the EOG. A dramatic decrease in muscle tone in the EMG and a desynchronized EEG distinguish rapid eye movement (REM) sleep. Table 27–2 defines terms commonly used in sleep studies.
| Term | Definition |
|---|---|
| Polysomnography (PSG) | Multi channel physiologic recording of sleep |
| REM sleep | Rapid eye movement sleep, characterized by bursts of rapid eye movements, low-voltage fast EEG, and atonia; associated with dreaming |
| NREM sleep | Non-Rapid eye movement sleep; consists of four stages |
| Stage 1 | A transitional state of lighter sleep between wakefulness and full sleep; characterized by low voltage, mixed frequency EEG and slow rolling eye movements |
| Stage 2 | Sleep characterized by EEG waveforms called K-complexes and spindles, usually around 50–75% of TST |
| Stage 3 | Sleep characterized by 20–50% high amplitude slow EEG waves (25–50%) |
| Stage 4 | Sleep characterized by > 50% high amplitude slow EEG waves |
| Total sleep time (TST) | Total minutes of NREM sleep + total minutes REM sleep |
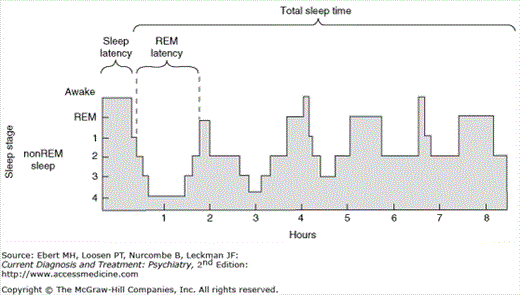
| Sleep latency | Elapsed time from “lights out” to onset of sleep |
| REM latency | Elapsed time from sleep onset to REM onset; normally varies from 70–100 min (in young adults) to 55–70 min (in elderly); may be abnormally short in narcolepsy, depression and other conditions |
| Sleep efficiency (SE) | Time asleep divided by total time in bed; usually expressed as a percentage, normally ≥ 90% in young adults; decreases somewhat with age |
| Wakefulness after sleep onset (WASO) | Time spent awake after sleep onset |
| Respiratory disturbance index (RDI) | Respiratory events (apneas + hypopneas) per hour of sleep; sometimes referred to as apnea-hypopnea index (AHI) |
| Apnea | A cessation of airflow of 10 s or longer |
| Hypopnea | A reduction by 50% in airflow of 10 s or longer |
| Multiple Sleep Latency Test (MSLT) | An objective measure of EDS in which sleep latency and REM latency are measured during four to five 20-min nap opportunities spaced 2 h apart across the day |
| Periodic Limb Movements (PLMs) | Intermittent (every 20 –40 s) leg jerks or leg kicks during sleep |
Normal sleep involves two states: REM sleep and non-REM sleep. REM sleep is often associated with dreaming. Non-REM sleep is a period of decreased physiologic and psychological activity and is further divided into stages 1, 2, 3, and 4 on the basis of visually scored EEG patterns.
Sleep normally begins with non-REM stage 1, before progressing successively into non-REM stages 2 through 4, during which the EEG generally declines in frequency and increases in amplitude. Stages 3 and 4 sleep, also called slow-wave sleep (SWS), are typically most intense early in the sleep period. The amount of SWS declines across the night. REM sleep is characterized by high-frequency; low-amplitude EEG, loss of muscle tone in the major antigravity muscles, and REMs (see Figure 27–2).
The neurophysiologic underpinnings of sleep and wakefulness are incompletely understood. Aspects of REM sleep such as periodic REMs and atonia are generated within the brain stem. Non-REM sleep is partially controlled by rostral brain regions such as the hypothalamus, basal forebrain, and thalamus.
A variety of neurotransmitter systems and brain regions appear to regulate sleep and wakefulness. The arousal network involves the activity of neurons containing acetylcholine, norepinephrine, serotonin, orexin (hypocretin), and dopamine (DA), whereas Gamma aminobutyric acid (GABA)-ergic mechanisms figure prominently in initiating non-REM sleep (Table 27–3).
| Substance | Involved in Control of Wakefulness |
|---|---|
| Acetylcholine | Cholinergic neurons of the dorsal midbrain and pons densely innervate the thalamus and thus regulate alertness and cortical activation. |
| Serotonin | Dorsal raphe neurons (DRN) are active during waking, less active during NREM sleep, and virtually inactive during REM sleep. |
| Norepinephrine | Noradrenergic neurons in locus coeruleus (LC) are very active during waking, and are thought to promote wakefulness. These neurons cease firing in REM sleep. |
| Dopamine | Extracellular levels of DA are elevated during waking. D1 and D2 antagonists (typical antipsychotics) tend to promote sleep. |
| Histamine | Histaminergic neuronal activity is high during wakefulness, and H1 antagonists produce drowsiness and sleep. |
| Hypocretin/orexin | Orexin neurons are most active during waking and locomotor activity. Deficiencies in this system are presumed to cause narcolepsy. |
| Involved in control of NREM or REM sleep. | |
| GABAergic | Preoptic anterior hypothalamic neurons appear to promote sleep by inhibiting wakefulness via GABAergic projections. Hypnotic effects of BZDs may be mediated by enhancement of GABA. |
| Melatonin | Melatonin is secreted by the pineal gland and is best established as a marker of circadian rhythm. MEL may hasten sleep or ease jet lag. |
| Interleukins and other immune modulators | Interleukins promote SWS in animals, and immune modulators may be increased in plasma at sleep onset in normal control subjects. NREM sleep measures may correlate with natural killer cell activity in humans. |
| Adenosine | Adenosine appears to promote sleep. Alerting effects of caffeine may be mediated by blockade of adenosine receptors. |
| Serotonin | l-tryptophan has hypnotic effects, increases delta sleep. Serotonergic neurons in DRN cease firing in REM sleep and may inhibit cholinergic neurons in laterodorsal tegmentum-pedunculopontine tegmentum (LDT-PPT), ponto-geniculo-occipital (PGO) waves, and REM sleep. |
| Prostaglandins | Prostaglandins D2 and E2 increase sleep and wakefulness, respectively, in animals. |
| Endogenous sleep factors | Putative hypnotoxins include delta sleep inducing peptide (DSIP), uridine, arginine vasotocin, and muramyl peptides. |
The rhythm of sleep and wakefulness is governed by one or more internal biological “clocks,” by environmental stimuli, and by a host of processes that promote or inhibit arousal (see Table 27–4.) In the absence of zeitgebers (time cues such as social activities, meals, and bright lights), humans tend to self-select a sleep–wake cycle of about 25 hours from wake time to wake time. In other words, if a person lives in an experimental environment free of time cues and is allowed to go to bed and arise at will, that person will tend to go to sleep about an hour later each “night” and wake up about an hour later each “morning.” For this reason, shifts in the sleep–wake cycle activity are usually easier when the cycle is lengthened rather than shortened—in traveling west rather than east, for example—or when rotating from an afternoon to an evening work shift, rather than from an afternoon to a morning work shift.
| Term | Definition |
|---|---|
| Chronobiology | The Study of Circadian Rhythms |
| Circadian rhythms | Refers to biological rhythms having a cycle length of about 24 h. Derived from Latin: circa dies, “about 1 day.” Examples include the sleep–wake cycle in humans and temperature, cortisol, and psychological variation over the 24-h day. Characterized by exact cycle length, amplitude, and phase position. |
| Phase position | Temporal relationship between rhythms or between one rhythm and the environment. For example, the maximum daily temperature peak usually occurs in the late afternoon. |
| Phase-advanced rhythm | Patient retires and arises early. |
| Phase-delayed rhythm | Patient retires and arises late. |
| Zeitgebers | Time cues such as social activities, meals, and bright lights. |
Normally, the circadian oscillator is entrained to the 24-hour environment by zeitgebers such as social activities and meals, and especially by environmental light. Information about light reaching the retina is conveyed to the suprachiasmatic nuclei (SCN) in the anterior hypothalamus. The SCN are important oscillators that maintain the circadian rhythm of sleep–wakefulness.
In addition to synchronizing the circadian oscillator with the environment, the timing of light exposure can also shift the phase position of the oscillator (i.e., the temporal relationship between rhythms or between one rhythm and the environment). Bright light (1500 lux) in the evening hours (6–9 pm) coupled with darkness from 9 pm to 9 am tends to cause a phase delay in sleep–wake and other biological oscillators (i.e., one would go to bed later and wake up later). In contrast, exposure to bright light in the early morning hours (5–7 am) coupled with darkness in the evening tends to advance the phase position of the oscillator (i.e., one would go to bed earlier and wake up earlier). Furthermore, bright light during daylight hours can enhance the amplitude of the circadian rhythm, thereby demarcating the periods of both nocturnal sleep and daytime wakefulness. Bright light has been reported to have antidepressant effects in seasonal depressions occurring in the winter and in some patients with major depressive disorder or premenstrual depression.
Sleep–wake states change dramatically across the life span, not only with regard to the amount of sleep, but also to circadian timing. With advancing age, REM latency tends to decrease and the length of the first REM period tends to increase.
The amount of time spent each night in SWS is high in childhood, peaks in early adolescence, and gradually declines with age until it nearly disappears around the sixth decade of life. Young adults typically spend about 15–20% of total sleep time (TST) in SWS. Sleep tends to be shallower, more fragmented, and shorter in duration in middle-aged and elderly adults compared to young adults. In addition, daytime sleepiness increases. The relative amount of “shallower” stages 1 and 2 sleep tends to increase as the “deeper” stages 3 and 4 sleep tend to decrease. Men tend to lose SWS at an earlier age than women do.
After the age of 65, about one in three women and one in five men report that they take over 30 minutes to fall asleep. Wakefulness after sleep onset (WASO) and number of arousals increase with age, an increase that may be due at least in part to the greater incidence of sleep-related breathing disorders, PLMS, and other physical conditions in these age groups. WASO may also increase with age because older people are more easily roused by internal and external stimuli.
Changes in the circadian rhythm may lead to daytime fatigue, napping, and poor nocturnal sleep. Related to a phase-advanced temperature rhythm, elders tend to retire and arise earlier than younger adults. Psychosocial alterations can disrupt zeitgebers and light exposure. Napping also increases with age, but the TST per 24 hours does not change with age.
This section follows the system in the International Classification of Sleep Disorders, Revised (ICSD-2). which groups sleep complaints by primary symptomatology: Insomnia, or disorders of initiating and maintaining sleep; hypersomnia, or disorders of EDS; parasomnias; and circadian rhythm disorders. The section also comprises of a brief discussion of sleep alterations associated with psychiatric disorders, substance use, medical conditions, and the reproductive cycle.
Insomnia
DSM-IV-TR Diagnostic Criteria 307.42
Primary Insomnia
The predominant complaint is difficulty initiating or maintaining sleep, or nonrestorative sleep, for at least 1 month.
The sleep disturbance (or associated daytime fatigue) causes clinically significant distress or impairment in social, occupational, or other important areas of functioning.
The sleep disturbance does not occur exclusively during the course of Narcolepsy, Breathing-Related Sleep Disorder, Circadian Rhythm Sleep Disorder, or a Parasomnia.
The disturbance does not occur exclusively during the course of another mental disorder (e.g., Major Depressive Disorder, Generalized Anxiety Disorder, a delirium).
The disturbance is not due to the direct physiological effects of a substance (e.g., a drug of abuse, a medication) or a general medical condition.
(Reprinted, with permission, from Diagnostic and Statistical Manual of Mental Disorders, 4th edn. Text Revision. Copyright 2000 American Psychiatric Association.)
Insomnia is the complaint of difficulty initiating or maintaining sleep or of nonrestorative sleep (not feeling well-rested after sleep that is adequate in amount). Insomnia is more common in women than in men; more common with age; and often associated with medical and psychiatric disorders or use of alcohol, drugs, and medication.
Transient insomnia is much more common than chronic (> 1 month) insomnia. It generally results from acute stress. Many such cases resolve without intervention. PSG abnormalities have been documented in acute bereavement. However, persistent insomnia should raise the consideration of depression, adjustment disorder, or other psychiatric disorders. Psychophysiologic insomnia is a “disorder of somatized tension and learned sleep-preventing associations that result in a complaint of insomnia” (ICSD-2 1997). All patients with chronic insomnia probably develop learned sleep-preventing associations, such as marked overconcern with the inability to sleep. The frustration, anger, and anxiety associated with trying to sleep or maintain sleep serve only to arouse them further as they struggle to sleep. These patients can acquire aversive associations with their bedrooms, often sleeping better in other places such as in front of the television set, in a hotel, or in the sleep laboratory. Psychophysiologic insomnia can become chronic.
Because chronic insomnia is so commonly caused by medical, psychiatric, or substance use comorbidity or in association with medication, the clinician should always look carefully for other conditions and treat the primary disorder. Although 780.59 Breathing-Related Sleep Disorder is classified as a hypersomnia, apneic episodes can cause insomnia. Insomnia can also be associated with sleep-related movement disorders, for example, restless legs syndrome (RLS) and PLMS (discussed separately).
Nonpharmacologic treatment includes education about sleep hygiene (see Table 27–5, Stimulus-Control Treatment) as well as identifying and correcting faulty beliefs, for example, the fear of not being able to function at all without 8 hours uninterrupted sleep.
| Keep bedtimes and awakening constant, even on the weekends. |
| Do not use the bed for watching television, reading a book, or working. If sleep does not begin within a period of time, say, 30 min, leave the bed and do not return until drowsy. |
| Avoid napping. |
| Exercise regularly (3–4 times per week), but try to avoid exercising in the early evening if this tends to interfere with sleep. |
| Discontinue or reduce alcohol, caffeine, cigarettes, and other substances that may interfere with sleep. |
| “Wind down” before bed with quiet or relaxing activities. |
| Maintain a cool, comfortable, and quiet sleeping environment. |
Sleep restriction therapy involves gradually improving sleep consolidation (minimizing interruptions of the nocturnal sleep period) by limiting the time patients spend in bed. Many insomniac patients underestimate actual sleep time (“sleep state misperception”) and have poor sleep efficiency (SE). If the patient reports sleeping 6 hours per night, he or she is required to limit time in bed to 6 hours or slightly more. This simple maneuver usually produces mild sleep deprivation, shortens sleep latency, and increases SE. As sleep becomes more consolidated, the patient is allowed gradually to increase time in bed. It may be helpful to counsel for acute stressors and break the “vicious cycle” of psychophysiological insomnia.
Benzodiazepines (BZDs) have been the most widely prescribed true sedative-hypnotics, being safer than barbiturates. They generally reduce sleep latency, minutes awake after sleep onset, SWS, and REM while increasing Stage 2. The choice of BZD depends on onset and duration of action (in relation to the timing of sleep complaints) and anxiolytic properties if needed. In the absence of substance abuse history or concomitant abuse of other substances, short-term use of BZDs to treat insomnia is usually safe and effective. The long-term efficacy is not clear; physiologic tolerance can occur.
Non-BZD hypnotics include zolpidem, zaleplon, eszopiclone (the S-isomer of zopiclone), and ramelteon (see Table 27–6.) Compared to BZDs, these drugs tend to have less risk of misuse, rebound insomnia, and withdrawal symptoms and can generally be given to recovering addicts. Zolpidem can be taken in doses larger than described. Aside from ramelteon, these are GABAA receptor agonists, which probably explains their less marked motor and cognitive side effects. Onset and duration of action should be considered. Some nonBZDs can cause morning “hangovers” if taken too late in the night. Controlled-release zolpidem, is now The U.S. Food and Drug Administration (FDA) approved and has shown efficacy in long-term use as well.
| Generic | Trade | Half-life (h) | Onset | Dose, Adult (mg) | Mechanism |
|---|---|---|---|---|---|
| Zolpidem | Ambien | 1.5–2.4 | Fast | 5–10 | GABAA Agonist |
| Zaleplon | Sonata | 1 | Fast | 5–10 | GABAA Agonist |
| Eszopiclone | Lunesta | 5–7 | Medium | 2–3 | GABAA Agonist |
| Ramelteon | Rozerem | 1–2.6 | Fast | 8 | Melatonin Agonist |
Ramelteon, a selective melatonin (MEL) agonist (active at MT1 and MT2 sites), does not bind to GABA receptors, nor does it possess activity within the brain reward system. It is being marketed as “addiction proof”; if this claim stands the test of time, it will provide an important option for many patients in recovery. Its rapid onset of action and melatonergic mechanisms appear promising for initial insomnia, especially in the context of a delayed circadian phase.
Other medications prescribed for insomnia in the absence of psychiatric comorbidity include trazodone, other sedating antidepressants, and the more sedating atypical antipsychotics.
Over-the-counter (OTC) sleeping pills usually consist of or contain histamine 1 antagonists (e.g., diphenhydramine). Their efficacy is dubious. “Natural” remedies include valerian and MEL, the latter which has been used for many years and probably does have some efficacy.
The liability for tolerance, withdrawal, and abuse must be considered in regard to all the BZDs, although many patients with anxiety disorder and insomnia take them long-term without misuse, particularly after proper patient education and supervision. However, withdrawal from prolonged high-dose BZDs can cause seizures, psychosis, delirium or even death. Rebound insomnia can also occur even with well-planned tapering.
The “war on drugs” increasingly leads many clinicians to prescribe medication with less favorable safety profiles than BZDs, (e.g., risk of priapism with trazodone and metabolic complications with atypical antipsychotics). Elderly patients are particularly vulnerable to the anticholinergic side effects of antihistamines.
Many people think “natural” products are safer, not knowing that the FDA classifies them as dietary supplements and does not regulate them as closely as “manufactured” pharmaceuticals. Serious problems have resulted from unsafe processing (e.g., l-tryptophan byproducts causing an eosinophilia-myalgia syndrome). Recently, an analysis of MEL tablets bought at “reputable” pharmacies, supermarkets, and health food stores found widely varying actual doses of MEL as well as adulterants such as BZDs. Potential drug interactions are less well-known for complementary medicine products, particularly in regard to botanicals which contain multiple chemical compounds.
Restless Legs Syndrome/PLMS in Sleep
Insomnia associated with RLS, PLMS, or other sleep-related movement disorders is coded as 307.47 Dyssomnia Not Otherwise Specified.
DSM-IV-TR Criteria 307.47
Dyssomnia Not Otherwise Specified
The Dyssomnia Not Otherwise Specified category is for insomnias, hypersomnias, or circadian rhythm disturbances that do not meet criteria for any specific Dyssomnia.
(Reprinted, with permission, from Diagnostic and Statistical Manual of Mental Disorders, 4th edn. Text Revision. Copyright 2000 American Psychiatric Association.)
Restless leg syndrome (RLS) and PLMS are often discussed together because they overlap in presentation and symptoms. RLS is an uncomfortable “creeping, crawling” sensation or “pins and needles feeling” (described as similar to akathisia by patients who have had both) in the limbs, especially in the legs. RLS tends to occur during waking and at sleep onset, whereas PLMS occurs during sleep. Patients with RLS sometimes also have PLMS, but patients with PLMS often do not have RLS. For most patients with RLS, being recumbent increases leg discomfort and leads to difficulty sleeping. Further sleep disruption may occur if movement of the affected limb becomes the only way to relieve the dysesthesia.
PLMS are involuntary, rhythmic (roughly every 20–40 seconds over periods up to hours) twitches, typically ankle dorsiflexion. Each movement may lead to a brief arousal; PLMS can provoke tremendous sleep fragmentation, yet patients commonly are not consciously aware of the movements and may present with hypersomnia alone. They may present after accidentally kicking their bedmates or drastically disarranging the bed linens. PLMS increases with age, the prevalence being about 30% over 50 years and 50% over 65.
The differential diagnosis of RLS and PLMS includes akathisia, neurologic diseases (e.g., neuropathies, myelopathies, spinal cord problems) as well as systemic illness (e.g., anemia, nutritional/metabolic disturbances, cancer, and particularly chronic renal disease with dialysis). Similar symptoms can result from the discontinuation of illicit substances or medications, particularly antidepressants.
Treatment should include correcting underlying disorders (e.g., iron deficiency anemia) and (if possible) discontinuing the medications which cause RLS and PLMS. Most treatments reduce either the muscle activity or the sleep disruption. Treatment generally involves one of three drug categories: Dopaminergic agents (ropinirole, pramipexole); GABAergic agents (e.g., baclofen, gabapentin and other anticonvulsants, and BZDs, especially clonazepam); and opioids such as propoxyphene or codeine preparations (e.g., Tylenol 3). It is not uncommon to have to switch from one drug class to another after a previously effective medication loses efficacy; conversely, it can be helpful to switch to a previously effective medication in a different drug class.
Some sleep experts consider dopaminergic agents the treatment of choice for RLS, although their long-term effects have not been well studied, even in nonpsychiatric populations. Ropinirole (the only FDA-approved RLS treatment) and pramipexole (previously used in Parkinson’s disease [PD]) have more benign side effects than older agents (e.g., levodopa/carbidopa), and fewer peripheral side effects. Patients should be encouraged to use BZDs or opioids on alternate nights if possible. Anticonvulsants may be an option for recovering substance abusers, particularly if psychiatric comorbidity (e.g., psychosis) is a relative contraindication to dopaminergic agents.
Hypersomnias
DSM-IV-TR Diagnostic Criteria 780.59
Breathing-Related Sleep Disorder
Sleep disruption, leading to excessive sleepiness or insomnia, that is judged to be due to a sleep-related breathing condition (e.g., obstructive or central sleep apnea syndrome or central alveolar hypoventilation syndrome).
The disturbance is not better accounted for by another mental disorder and is not due to the direct physiological effects of a substance (e.g., a drug of abuse, a medication) or another general medical condition (other than a breathing-related disorder).
Coding note: Also code sleep-related breathing disorder on Axis III.
(Reprinted, with permission, from Diagnostic and Statistical Manual of Mental Disorders, 4th edn. Text Revision. Copyright 2000 American Psychiatric Association.)
DSM-IV-TR Diagnostic Criteria 347.00
Narcolepsy
Irresistible attacks of refreshing sleep that occur daily over at least 3 months.
The presence of one or both of the following:
cataplexy (i.e., brief episodes of sudden bilateral loss of muscle tone, most often in association with intense emotion)
recurrent intrusions of elements of rapid eye movement (REM) sleep into the transition between sleep and wakefulness, as manifested by either hypnopompic or hypnagogic hallucinations or sleep paralysis at the beginning or end of sleep episodes
The disturbance is not due to the direct physiological effects of a substance (e.g., a drug of abuse, a medication) or another general medical condition.
(Reprinted, with permission, from Diagnostic and Statistical Manual of Mental Disorders, 4th edn. Text Revision. Copyright 2000 American Psychiatric Association.)
DSM-IV-TR Diagnostic Criteria 307.44
Primary Hypersomnia
The predominant complaint is excessive sleepiness for at least 1 month (or less if recurrent) as evidenced by either prolonged sleep episodes or daytime sleep episodes that occur almost daily.
The excessive sleepiness causes clinically significant distress or impairment in social, occupational, or other important areas of functioning.
The excessive sleepiness is not better accounted for by insomnia and does not occur exclusively during the course of another Sleep Disorder (e.g., Narcolepsy, Breathing-Related Sleep Disorder, Circadian Rhythm Sleep Disorder, or a Parasomnia) and cannot be accounted for by an inadequate amount of sleep.
The disturbance does not occur exclusively during the course of another mental disorder.
The disturbance is not due to the direct physiological effects of a substance (e.g., a drug of abuse, a medication) or a general medical condition.
Specify if:
Recurrent: If there are periods of excessive sleepiness that last at least 3 days occurring several times a year for at least 2 years.
(Reprinted, with permission, from Diagnostic and Statistical Manual of Mental Disorders, 4th edn. Text Revision. Copyright 2000 American Psychiatric Association.)
The term hypersomnia encompasses pathologically increased sleep duration (e.g., the patient with atypical depression who sleeps 14 hours a day), “sleep attacks” (abrupt involuntary onset of sleep), EDS, or a combination of these). It is important to note that hypersomnia or EDS can also be associated with poor nocturnal sleep (such as sleep disrupted by PLMs in sleep or other parasomnias) and with circadian rhythm disorders. The most common cause of EDS in the general population is chronic lack of sleep. It is important to differentiate between fatigue and EDS.
The treatment of hypersomnia depends on diagnosis. When possible, treatment should attempt to correct an aspect of the pathophysiology itself. Supportive therapy may help patients adjust to the illness and its social sequelae (e.g., being fired for falling asleep on the job).
Prognosis depends on the nature of the underlying disorder, neurological causes of hypersomnia other than narcolepsy being generally more difficult to treat. Early diagnosis and treatment is vital for all hypersomnias to minimize psychosocial sequelae and potentially fatal results (e.g., falling asleep at the wheel of a vehicle).