Overnight oximetry studies in three patients with suspected sleep disordered breathing. For each example, the top tracing shows the oxygen saturation readings associated with values on the left vertical axis, and the bottom tracing shows the heart rate readings associated with values on the right vertical axis. A. Normal study, with oxygen saturation readings above 90 % and no significant oxygen saturation drops over the course of the study. B. Abnormal study, with oscillatory saw-toothed waveforms with variable degrees of desaturation, is highly suggestive of obstructive sleep apnea (OSA). Note the increases in heart rate during periods of the most consistent periodic desaturations. C. Abnormal study, with a relatively regular pattern of recurrent oxygen desaturation highly suggestive of central sleep apnea (CSA).
Management. Management of CSA can be challenging. Nasal CPAP therapy often worsens CSA. For many years, bi-level PAP therapy with or without supplemental oxygen was efficacious for many patients. Over recent years, adaptive servoventilation (ASV) has gained increased utility in CSA management – provided that certain criteria on PSG are satisfied.
Complex sleep apnea syndrome
In those patients with OSA who develop a high frequency of central apneas and/or a disruptive Cheyne–Stokes respiratory pattern after application of CPAP, this is termed “complex sleep apnea syndrome” (CompSAS) [35, 36]. The incidence and prevalence of CompSAS in patients with dementia have not been well studied, but this disorder is present in the dementia population.
Diagnosis. CompSAS is diagnosed on PSG when central apneas occur with high frequency associated with CPAP for obstructive apneas.
Management. ASV has been shown to be particularly effective in treating CompSAS [37].
Sleep-related movement disorders
Restless legs syndrome
Restless legs syndrome (RLS) is common in the population, and the prevalence increases with age.
Diagnosis. The diagnosis of RLS is a clinical diagnosis and is purely based on a patient’s symptoms. Typical descriptors of the symptoms include “creepy-crawly,” “tingly,” “gnawing,” or “pins and needles,” etc. The cardinal features of RLS are (1) presence of uncomfortable sensations in the legs associated with the strong urge to move the legs, (2) symptoms are worse when at rest, (3) symptoms improve temporarily with leg movement, and (4) symptoms are worse at night – particularly when lying down. A PSG is not required or even needed to make a diagnosis of RLS in a patient regardless of whether a coexisting neurologic syndrome is present or not.
Since the diagnosis of RLS is based on the history, those with cognitive impairment may have difficulties recalling or describing their symptoms. Yet many are able to voice some symptoms that raise suspicion of RLS, and a spouse or other informant who knows the patient well is often able to provide some descriptions such as “he/she seems to be restless or move the legs when sitting and/or at night.”
Management. Some patients find that warm baths in the evening can minimize RLS symptoms. Clinicians must be mindful of the association of iron deficiency and RLS; when these coexist, it is often very difficult to effectively treat RLS. Results from a standard complete blood count are often normal – it is the ferritin level that is most important. Those with RLS and ferritin levels below 50 ng/ml typically have limited responses to therapy. If a low level is present, clinicians should work with the patient’s primary care provider to determine if a work-up for identifying the source of iron deficiency, which is usually due to gastrointestinal blood loss (e.g., ulcer, gastritis, colon polyp, diverticular disease or adenocarcinoma), inadequate iron ingestion in the diet, or poor absorption from prior gastric surgery. A combination of ferrous fumarate plus vitamin C is usually effective and well tolerated, but improvement may not be apparent for at least a month. In some, one or more iron infusions are needed to normalize ferritin levels and provide RLS relief.
Many patients with RLS experience troublesome symptoms every night, are rather miserable, and insomnia associated with RLS is common. Pharmacotherapy is warranted in patients with ongoing symptoms despite non-drug therapies having been utilized. Several agents (e.g., carbidopa/levodopa, pramipexole, ropinirole, rotigotine, and gabapentin) are generally well tolerated and efficacious. Dopaminergic agents can aggravate psychosis, and therefore, gabapentin may be preferred in those with problematic hallucinations and/or delusions.
Periodic limb movements during sleep
Periodic limb movements during sleep (PLMS) refers to brief movements of one or both legs – typically flexion of the anterior tibialis plus other flexor muscles of the lower limb – which occur in periodic fashion (typically every 15 to 90 seconds) during sleep. The term “periodic limb movement disorder” (abbreviated PLMD) refers to a similar process, but since there are controversies relating to the cut-off of PLMS to be considered a “disorder,” and even if this is a clinically significant issue in most patients, the term periodic limb movements during sleep (and abbreviation PLMS) is used in this chapter.
Diagnosis. The bedpartner must be queried to gain insights into possible PLMS, and if PLMS are suspected to be disrupting the patient’s sleep, a PSG may be warranted. Some patients report that their legs jerk while attempting to fall asleep at night, and while these can be indeed bothersome (and often co-occur in those with RLS), they are technically not PLMS per se as they are occurring during wakefulness and not sleep. Also, some bedpartners are not aware of any “leg jerks” or “leg movements” during sleep at all, and yet on PSG, PLMS occurring 100–200 times per hour can be documented. A PSG is required to be confident in the presence and frequency of PLMS.
Some unresolved issues are what frequency of PLMS, and what frequency of arousals due to PLMS, are clinically significant. One reasonable approach is to consider that one night’s PSG provides an approximation of the nightly presence and frequency of key sleep indices, so it is probably not necessary to rely on hard cut-offs for PLM frequency and PLM arousal frequency to make diagnostic and treatment decisions. In other words, if a patient has at least 30 PLMS per hour, particularly if a high percentage is associated with arousals, then such patients might have clinically significant PLMS and therapy may be justified.
Management. As in RLS, treatment with carbidopa/levodopa, pramipexole, ropinirole, rotigotine or gabapentin is generally efficacious and well tolerated. Again, the dopaminergic agents should be used with caution in patients with psychotic features as these agents can aggravate psychosis. Measuring ferritin may also be worthwhile to ensure relative iron deficiency is not contributing to PLMS; for values below 50 μg/ml, iron replacement therapy is reasonable.
Sleep related leg cramps
Sleep related leg cramps (SRLC), otherwise known as “nocturnal leg cramps” or “Charlie horse” cramps, can impact the ability to fall and remain asleep. SRLC represent painful involuntary muscle contractions, and are relatively common in the population. The gastrocnemius is usually involved, although the intrinsic foot muscles or anterior tibialis muscle or even intrinsic hand muscles are sometimes affected. SLRC are also more common in those who have disorders that affect neuromuscular integrity such as peripheral neuropathies, lumbosacral radiculopathies, etc. It is not uncommon for fasciculations to be present in the gastrocnemius muscles on neurologic examination. They can also occur when acetylcholinesterase inhibitors are dosed in the evening.
Diagnosis. The diagnosis is based on the clinical history alone – gathered from the patient and/or bedpartner. Clinical evidence of a peripheral neuropathy, lumbosacral radiculopathy, fasciculations, etc., additionally supports the suspicion.
Management. Quinine sulfate was the mainstay of therapy until the FDA ordered all unapproved drugs containing quinine off the market several years ago. While controlled studies are minimal, therapies used in the management of SRLC include consumption of one or more of the following before bedtime: tonic water (which has dilute quinine in it), bananas, calcium-containing antacids, levodopa, dopamine agonists, and anticonvulsants, among many other prescription and non-prescription agents. Clinical experience has shown that nightly use of 4–6 ounces of tonic water can be quite effective in many patients, and is generally well tolerated.
Circadian rhythm sleep disorders
Several groups have observed a high frequency of sleep fragmentation and circadian rhythm sleep disorders (also known as “circadian dysrhythmias”) in the institutionalized elderly [38–41]. Sleep histories and sleep logs must obviously be gathered from bedpartners or caregivers with knowledge of the patient’s sleep schedules.
There is also evidence from animal and human studies that alterations in sleep continuity can disrupt the diurnal pattern and amplitude of beta amyloid in the brain, thereby potentially contributing to AD pathogenesis [42, 43]. Orexin appears to have a key role in this process [42]. Degeneration of the suprachiasmatic nucleus and the effects on melatonin production and secretion contribute to circadian dysrhythmias and possibly amyloid physiology [44]. Drugs which promote and maintain slow-wave sleep, including but not limited to orexinergic agents, as well as melatonin agonists, could improve sleep quality/continuity as well as potentially decrease the risk of the development of AD.
Advanced sleep phase syndrome
The advanced sleep phase syndrome (ASPS) refers to the circadian rhythm being phase-advanced such that the tendency to fall asleep is earlier in the evening (e.g., 6 pm to 9 pm) and the wake onset is earlier in the morning (e.g., 2 am to 5 am). ASPS occurs with increased frequency in the aged population.
Diagnosis. A detailed sleep history +/- a sleep log can usually provide sufficient data to suspect ASPS, with sleep onset times and wake onset times occurring in the ranges noted above. When a diagnosis is not clear, wrist actigraphy can provide useful data.
Management. Modest physical activity and particularly phototherapy (with at least 45 minutes of either adequate natural sunlight or 10,000 lux via a lightbox) exposure in the late afternoon or early evening are the mainstays of treatment. Low-dose methylphenidate (2.5 to 10 mg) in the late afternoon can also be helpful.
Delayed sleep phase syndrome
The delayed sleep phase syndrome (DSPS) refers to the circadian rhythm being phase-delayed such that the tendency to fall asleep is later in the night (e.g., 12 am to 3 am) and the wake onset is later in the morning (e.g., 8 am to 11 am). DSPS is much more common in the younger population and rare in the elderly.
Diagnosis. A detailed sleep history +/- sleep log and actigraphy are useful to establish a diagnosis of DSPS.
Management. Treatment involves using sedative/hypnotics to promote sleep in a desirable time frame in the evening and phototherapy (with at least 45 minutes of 10,000 lux via a lightbox) in the morning.
Irregular sleep–wake circadian dysrhythmia
The irregular sleep–wake circadian dysrhythmia (also known as the circadian rhythm sleep disorder – irregular sleep–wake type) is relatively frequent in the dementia population, which is likely due to degenerative changes in the suprachiasmatic nucleus of the hypothalamus and decreased melatonin production.
Diagnosis. As in the other circadian dysrhythmias, a detailed sleep history +/- sleep log and actigraphy are important.
Management. The primary management strategies involve exogenous melatonin and phototherapy. Data from relatively small numbers of patients with dementia suggest that melatonin can improve sleep continuity and lengthen total sleep time [45, 46]. A multicenter placebo-controlled trial using 2.5 mg slow-release and 10 mg formulations of melatonin failed to demonstrate efficacy over placebo, although there were individual patients who clearly appeared to benefit from melatonin [47]. Studies with ramelteon – am M1/M2 (melatonin) receptor agonist – for management of circadian dysrhythmia in patients with dementia are in progress, and clinical experience thus far has yielded variable efficacy but good tolerability. A similar agent – tasimelteon – has been FDA approved for non-24 hour sleep–wake disorder; no studies have been published to date in those with dementia and circadian dysrhythmias. Phototherapy has shown some promise in the management of circadian dysrhythmia in demented individuals, although the optimal timing and duration of phototherapy and illumination intensity is a matter of debate [48–51]. A new class of agents – the orexin receptor antagonists – will surely be studied in patients with insomnia associated with dementia.
Hypersomnias of central origin
The hypersomnias of central origin refer to narcolepsy, idiopathic hypersomnia, and hypersomnia due to an underlying medical/neurologic disorder.
The onset of narcolepsy and idiopathic hypersomnia typically begins in the teenage or early adult years; these tend to be already diagnosed years prior to the onset of cognitive decline. Yet these must always be a diagnostic consideration in anyone with a hypersomnolence syndrome when no other cause can be identified, particularly if other features of narcolepsy are present (e.g., cataplexy, sleep paralysis, hypnogogic/hypnopompic hallucinations).
The most common hypersomnia of central origin in those with dementia is due to the underlying cause of the dementia syndrome. In those with vascular dementia, infarcts particularly in the thalamus are associated with hypersomnia. The underpinnings of hypersomnia in neurodegenerative disorders are unclear, although alterations in hypocretin production or physiology may be at play [52].
Clinical experience indicates some individuals with dementia with Lewy bodies or frontotemporal dementia have objective evidence of hypersomnolence as measured by the Multiple Sleep Latency Test, and/or impairment in the ability to maintain wakefulness as measured by the Maintenance of Wakefulness Test (MWT). Recent evidence has revealed hypersomnia as measured by the MSLT in DLB patients compared to those with AD [53].
Diagnosis. A detailed sleep history +/- sleep log and actigraphy are helpful to evaluate the hypersomnias, and PSG followed by the MSLT is most helpful in characterizing the hypersomnolence syndrome. If the PSG shows (a) adequate total sleep time and (b) clinically insignificant sleep-disordered breathing and sleep-related movements, then the MSLT can support a central hypersomnia worthy of pharmacotherapy if the mean initial sleep latency is less than 10 minutes. PSG plus MSLT may not be feasible or practical in many patients with dementia, but if the degree of dementia is mild and long-term therapy may be needed, these diagnostic studies can be justified.
Management. Low-dose methylphenidate (5 mg or less) has been shown to improve alertness in very old patients residing in nursing homes [54], and there is very recent evidence suggesting methylphenidate in older individuals might reduce fall risk [55]. Modafinil, armodafinil [56], methylphenidate, dextroamphetamine, and dextroamphetamine/metamphetamine have been moderately helpful in managing hypersomnolence in patients with dementia without inducing untoward neurobehavioral or cardiovascular side effects.
Parasomnias
The most common and pertinent parasomnia in patients with dementia is REM sleep behavior disorder (RBD).
REM sleep behavior disorder
RBD is characterized by simple or complex limb movements and/or vocalizations during rapid eye movement (REM) sleep, and such behaviors typically mirror the content of the dream when a patient is awakened and questioned [57]. Behaviors can be violent, and patient and bedpartner injuries can occur. Readers are encouraged to review some key papers on this parasomnia [58–60].
As alluded to in the overview section above, the presence of RBD is providing a window into neurodegenerative diseases that few other symptoms or disorders have ever provided. The association of RBD with neurodegenerative disease is now well established, and it occurs particularly frequently in dementia with Lewy bodies (DLB), Parkinson’s disease with (PDD) or without dementia, multiple system atrophy, and pure autonomic failure (known collectively as the “synucleinopathies” [61–66]. Analyses have also shown that in those patients with dementia and RBD, the pattern of impairment on neuropsychological testing (e.g., impaired visuospatial functioning, verbal fluency, and attention/concentration, with relative preservation of memory and confrontational naming) is quite consistent, and parallel to what has been described in autopsy-proven Lewy body disease [67, 68]. RBD associated with dementia is highly predictive of underlying LBD [69]. Furthermore, all published patients who have had MCI plus RBD and have come to autopsy to date have had Lewy body disease pathology [70]. Therefore, a careful sleep history and neuropsychological testing may provide diagnostically relevant information in the evaluation of patients with mild cognitive impairment or dementia.
Studies involving patients with “idiopathic” RBD who have been followed prospectively have shown well over 80% of patients develop MCI, DLB, PD or MSA after 15 years from onset [71–73]. Therefore, there is considerable interest in focusing on those with RBD for future therapeutic trials in the hope of delaying the onset or preventing dementia and/or parkinsonism [58, 74–77].
Diagnosis. RBD is not a difficult disorder to suspect clinically. One simple question posed to a patient’s bedpartner usually provides a response that makes one not suspicious at all, or very confident that RBD is likely – “Have you ever seen the patient appear to ‘act out his/her dreams’ while sleeping? (punched or flailed arms in the air, shouted or screamed)?” Several questionnaires have been developed and validated to screen for RBD; one such measure that uses this question is described in these references [78, 79]. If the response is “no,” there is little need to question further. The bedpartners of those patients with RBD will state “yes” and then go on to describe a litany of experiences over many years which can be both comical and frightening, and patients usually report dreams of being chased or attacked. Importantly, some patients with moderate to severe OSA have historical features identical to RBD [80], and thus PSG should be performed when a confirmed diagnosis is needed for clinical purposes. In other words, when the diagnosis is in question, particularly when the potential for injury is present, polysomnography is warranted and preferably performed with additional EMG leads on the arms and with synchronous video/PSG monitoring.
Management. Ensuring safety in the sleep environment is the simplest recommendation for managing RBD (i.e., remove all potentially injurious objects away from the bed, place mattress on floor next to bed, etc.). Clonazepam and/or melatonin have the best track record in managing RBD [81]. Clonazepam has been the drug of choice and is usually effective in the 0.25 to 1.0 mg/night dose range [82, 83]. As benzodiazepines can precipitate or aggravate OSA, it is important to ensure that no OSA exists before using this agent, or ensure nasal CPAP therapy is successfully treating OSA if this is present. There is often concern expressed by clinicians about using this long-acting benzodiazepine in patients with dementia, but in our experience it has been tolerated quite well with few or no cognitive side effects in the vast majority of cases. Melatonin has also been shown to be effective at minimizing RBD, either by itself or in combination with clonazepam [84–86]. We typically commence 3 mg nightly, and depending on clinical response and side effects (which are typically rare), titrate upward in 3 mg increments every three to seven nights as needed and tolerated, up to 12 mg/night. In those rare individuals who have pervasive RBD which is not adequately responding to clonazepam plus melatonin, adding quetiapine can be effective. Some patients derive benefit from controlled-release carbidopa/levodopa for RBD, so in those with RBD plus RLS and/or PLMs, this agent may be a good first option.
Section 2: assessment tools
As in any area of clinical medicine, the history and physical examination usually provide most of the critical clues needed to determine which additional measures would be most useful in the diagnostic assessment process. In some cases, a firm diagnosis can be established by history and exam alone, and without the need for additional tests.
Similar to the assessment of those with dementia, in which interviewing the caregiver/informant is essential, the same is necessary in the assessment of sleep-related issues. A knowledgeable informant – preferably a bedpartner – is essential to interview. If a bedpartner is not present during a clinical interview session, it is incumbent on the clinician to telephone or communicate in some other manner with the bedpartner.
Many clinicians have also developed a series of key questions as part of written questionnaires, which patients and their bedpartners complete prior to the oral clinical interview. Such questionnaires permit an efficient way to screen for most of the primary sleep related symptoms and disorders. A list of representative questions is shown in Table 27.1. Key findings to elicit on physical and neurologic examination are shown in Table 27.2. Select questionnaires and other measures commonly in use are shown in Table 27.3.
Query patients and caregivers/bedpartners on the following:
Difficulties falling and staying awake
Loud disruptive snoring
History of appearing to “act out one’s dreams” such as punching or flailing arms in the air, shouting or screaming
Tendency to snort or choke during sleep
Tendency to stop breathing during sleep
Tendency to doze easily during the day
Tendency to experience unpleasant, nervous, creepy-crawly sensations in the legs/feet, primarily at night or when sitting at rest, an urge to move the legs, and the tendency for the unpleasant sensations to temporarily be relieved by moving the legs or walking
Tendency for the legs to periodically jerk during sleep
Tendency to experience cramps or “Charlie horse” episodes prior to or during sleep
Tendency to struggle falling asleep before 1–3 am, and then tendency to awaken after 8 am
Tendency to struggle maintaining wakefulness prior to 8 pm, and then tendency to awaken earlier than 6 am
Patency of oropharynx
Neck circumference
Degree of cognitive impairment (using mental status examination)
Presence of reduced vision (especially color vision)
Presence of reduced smell
Presence of parkinsonian findings
Presence of peripheral neuropathy findings
Presence of autonomic findings
Presence of fasciculations
Friedman:
http://www.ncbi.nlm.nih.gov/pubmed/10591345
Mallampati:
Pittsburgh Sleep Quality Index
http://www.sleep.pitt.edu/content.asp?id=1484&subid=2316
Mayo Sleep Questionnaire
http://www.mayoclinic.org/pdfs/MSQ-copyrightfinal.pdf
Epworth Sleepiness Scale
Sleep diary/sleep log
Sleep diaries, also known as sleep logs, are completed by patients (or their bedpartners) by writing sleep and wake related times on a sheet of paper. The patient records the estimated time of sleep onset, wake onset, as well as awakenings during the sleep period and naps during the otherwise wake period on a daily basis, usually over 1–2 weeks. These data inform the patient as well as his/her clinician if he/she tends to go to sleep in the early morning hours (e.g., after midnight) and wake late in the morning (e.g., after 8 am) which suggests delayed sleep phase syndrome, tends to go to sleep early in the evening (e.g., before 10 pm) and wake early in the morning (e.g., before 6 am) which suggests advanced sleep phase syndrome, tends to go to sleep late and wake early such that less than 7 or 8 hours is achieved which suggests insufficient sleep syndrome – a common situation in society – or tends to awaken for prolonged time segments during the sleep period. While some patients or their bedpartners may not record these sleep phenomena accurately for various reasons, sleep diaries can be a simple and useful measure for assessing several sleep disorders.
Wrist actigraphy
An actigraph is a device that is usually worn like a wristwatch (but can also be worn around the ankle). The accelerometer in the device measures limb movement. The relative frequency of limb movements is recorded over 24-hour period intervals, usually for 1- to 2-week periods. The graphical display of limb movements shows relative high frequencies of activity, which suggest wakefulness, and relativey low frequencies of activity, which suggest sleep. The abrupt cessation of activity during the evening hours suggests sleep onset, and the abrupt return of activity during the morning hours suggests wake onset. Periods of relative activity during the nocturnal hours suggests periods of wakefulness.
Actigraphy therefore usually offers more accurate readings of activity than patient or bedpartner reports in sleep logs. The disadvantages of conventional actigraphy included the cost required to purchase the device, cost of the computer hardware and software required to process and view the data (which is often printed for review and incorporating into patient medical records), the occasional device malfunctions, and the need to keep the device dry (and thus removal during bathing or swimming, and of course remembering to place the device back on after bathing or swimming). With the advent of smartwatches and other devices (e.g., Fitbit, Apple Watch, etc.), data similar to what has been obtained using conventional wrist actigraphy can now be gathered and analyzed with relative ease and low expense.
Overnight oximetry
Overnight oximetry involves patients placing a monitor on a finger or earlobe (finger is used most frequently) and maintaining this on the finger through the sleep period. The monitor measures oxygen saturation, which is then transmitted from the monitor to the oximeter meter via a wire. The meter records the oxygen saturation every 5–30 seconds. The standard output from overnight oximetry includes the percentage of oximetry readings in the 90–100%, 80–89%, 70–79%, etc., ranges over the total sleep time, the number of saturation drops of 4% or more during the total sleep time, and the tracings of the oxygen saturation as well as pulse recordings are displayed. The data are typically printed for review and incorporated into the patient’smedical record.
While the numerical data are important, the tracings are particularly informative. A tracing showing oscillatory saw-toothed waveforms with variable degrees of desaturation is highly suggestive of OSA. A tracing showing a relatively consistent pattern of saw-toothed waveforms with similar degrees of desaturation is more consistent with CSA. Examples of normal and abnormal overnight oximetry studies are shown in Figure 27.1.
Overnight oximetry does have its limitations. Those with respiratory associated respiratory arousals (often abbreviated RERAs) as part of the upper airway resistance syndrome (UARS) have, essentially by definition, almost normal or entirely normal oximetry studies. Also, those with normal cardiopulmonary functioning who are not obese can have essentially or completely normal overnight oximetry studies, yet PSG can demonstrate clinically significant OSA. There are therefore instances that PSG should still be performed if the index of suspicion of clinically significant OSA, or UARS, is high.
Overnight oximetry is a very useful screening measure in patients with the core features of sleep-disordered breathing. In view of the high prevalence of OSA in the middle-aged to elderly population, the link between some degree of cognitive impairment and OSA, and fact that OSA is treatable, clinicians should consider screening for OSA by history, exam, +/- overnight oximetry in every patient they evaluate for cognitive concerns.
Polysomnography
Polysomnography (PSG) is the formal name of an overnight sleep study. This involves monitoring devices which inform the PSG interpreter of which stage of sleep the patient is in over each period of measurement as well as other key normal and abnormal sleep-related phenomena. The monitoring devices typically include two electro-oculogram (EOG) derivations, three electroencephalography (EEG) derivations (Fz-Cz, Cz-Oz, C4-A1), electrocardiogram (ECG), chin and at least two limb surface electromyographic (EMG) electrodes, oronasal airflow, sonogram, oxyhemoglobin saturation, and chest and abdomen inductance plethysmography. The devices monitor sleep-related phenomena continually, which can be reflected on paper (rarely used anymore) or on computer screens for scoring. The standard period of PSG recording for scoring purposes is 30 seconds, which is also termed a “30 second epoch” or “30 second PSG fragment.”
The EOG derivations are important for demonstrating eyeblinks during wakefulness, slow eye movements as wakefulness begins to transition into stage N1 sleep (Stage 1 NREM sleep in the older terminology), and rapid eye movements (REMs) during Stage R sleep (REM sleep in the older terminology; the “REM sleep” term has been retained in this chapter). The EEG derivations demonstrate the defining characteristics and frequencies of EEG activity for the sleep stages. The ECG demonstrates the standard PQRST waveforms which are important for characterizing changes in heart rate in some sleep stages, and more importantly, changes in heart rate or rhythm or PQRST waveforms characteristic of pathologic ECG abnormalities. The downside of ECG recording as part of PSGs is that ECG artifact is evident on many derivations – particularly the surface EMG derivations – which can obscure the differentiation of some normal vs. abnormal sleep phenomena. Surface EMGs are typically placed over the submentalis muscle, over the extensor digitorum communis on one arm, and over the tibialis anterior on one or both legs (some sleep laboratories place surface EMGs on both arms and legs, or even more muscles, as part of their parasomnia protocol). These EMG leads are important for demonstrating loss of EMG tone as part of normal Stage R sleep, and three primary important abnormal sleep phenomena – (1) EMG activity reflecting movement which is often (but not always) associated with arousals, (2) periodic bursts of EMG activity particularly in the legs reflecting periodic limb movements, and (3) increased EMG tone +/- simple or complex motor behavior reflecting REM sleep without atonia (which is the electrophysiologic substrate of REM sleep behavior disorder). Oronasal airflow is self-explanatory; cessation of airflow reflects an obstructive, central or mixed apneic event (if of sufficient duration). The sonogram is basically a small microphone usually placed on the throat, and the features of the waveform generated from the sonogram reflect breathing phenomena such as snoring, stridor, vocalizations, etc. Oxyhemoglobin saturation is self-explanatory. Chest and abdomen inductance plethysmography measures changes in chest and abdominal circumference. During normal breathing, the chest and abdominal curves change in synchrony; during a central apneic event, the curves in both are flat; during an obstructive apneic event, the curves are in dysynchrony due to chest expansion and abdominal restriction during inspiration and the opposite occurring during expiration. An example of a normal 30 second epoch of PSG is shown in Figure 27.2A, and an example of an abnormal 30 second epoch demonstrating REM sleep without atonia is shown in Figure 27.2B.
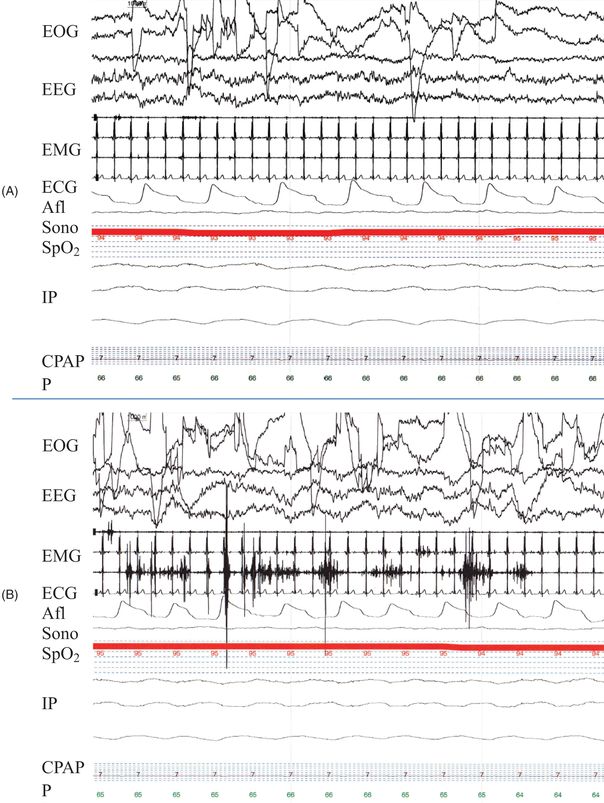
Polysomnogram fragments (30 seconds) in two patients. A. Normal fragment of REM sleep showing rapid eye movements (abrupt upward and downward deflections in the EOG leads), theta activity on the EEG leads, no EMG activity other than ECG artifact in the EMG leads, and normal breathing-related alterations in the Afl and IP leads. The patient is being administered nasal CPAP at 7 cm H20. B. Abnormal fragment of REM sleep showing increased EMG activity (i.e., REM sleep without atonia) in two of the three EMG leads. Video synchronized to the PSG recording demonstrated vigorous kicking movements of the legs corresponding to the increase EMG tone.
Key: EOG = electrooculogram (left and right outer canthus leads), EEG = electroencephalogram (Fz-Cz, Cz-Oz, and C4-A1 leads), EMG = electromyogram (submentalis, arm, and leg leads), ECG = electrocardiogram, AFl = airflow, Sono = sonogram, SpO2 = oxyhemoglobin saturation, IP = inductive plethysmography (sum of chest and abdomen, chest, and abdomen leads), CPAP = continuous positive airway pressure reading, P = pulse.
There are many facets to what is recorded, calculated, and reported in a standard PSG report. Many of these key parameters and PSG-related definitions are described below, along with “clinical pearls” which are stated after the term “Note:”
Sleep architecture
Time in bed (TIB) – total time spent in bed, which includes sleep and wakefulness.
Total sleep time (TST) – total time of sleep recorded over the PSG. Note: most PSGs are performed in the hope that at least 6–7 hours of TST will be recorded, but useful data can be acquired in many patients even with 2–3 hours of TST.
Sleep efficiency (SE) – total sleep time divided by the total time in bed multiplied by 100%. Note: SEs in sleep laboratories are highly variable, and this value is usually not given all that much weight in clinical practice unless the SE is very low or very high.
Sleep stage percentages – total time in each sleep stage (N1, N2, N3, and R) divided by TST multiplied by 100%. Note: the norms vary by age, but generally speaking, normal values are as follows: N1: 10–30%, N2: 40–60%, N3: 10–30%, R: 15–30%.
Wake after sleep onset (WASO) – total time of scored wakefulness that occurred after the initial epoch of sleep.
Sleep disordered breathing
Obstructive apneic event – a 10 second or longer period with clear amplitude decrease in breathing from baseline associated with > 3% oxygen desaturation or an arousal from the obstructive events.
Central apneic event – a reduction or absence of breathing and respiratory effort that lasted 10 seconds or longer, with associated reduced airflow.
Hypopnea event – a reduction of airflow and reduction of thoracic and/or abnormal movement that led to an arousal.
Respiratory effort related arousal (RERA) – a change in airflow, usually involving a snore, that led to an arousal; no reduction in airflow occurs in a RERA.
Respiratory disturbance index (RDI) – the sum of disordered breathing events related to obstructive apneas, central apneas, mixed apneas, hypopneas, and respiratory effort related changes averaged over the total sleep time, which represents a value of the sum per hour. Note: an RDI ≥ 5 is generally considered clinically significant and therefore abnormal.
Apnea/hypopnea index (AHI) – the sum of disordered breathing events related to obstructive apneas, central apneas, mixed apneas, and hypopneas averaged over the total sleep time, which represents a value of the sum per hour. Note: an AHI ≥ 5 is generally considered clinically significant and therefore abnormal. Also note that the distinguishing feature between the AHI and the RDI is that the RDI includes all parameters in the AHI plus RERAs.
Note: severity of sleep disordered breathing – generally speaking, AHI or RDI values in the 5–15 range are considered reflective of “mild” sleep disordered breathing, values in the 16–30 range are considered reflective of “moderate” sleep disordered breathing, values in the 31–60 range are considered reflective of “severe” sleep disordered breathing, and values > 60 are in the “very severe” category.
Movement-related phenomena
Periodic limb movement (PLM) – a periodic contraction of lower legs either unilateral or bilateral, with a series of four consecutive movements separated by 4 to 90 seconds, each movement lasted between 0.5 and 5 seconds, and not associated with respiratory events.
Periodic limb movement index (PLMI) – total number of PLMs per hour averaged over total sleep time.
Note: there are no generally accepted cut-offs for normal versus abnormal values for PLMI. PLMs are common in the population, and usually they are not clinically significant. However, the higher the PLMI, the more likely that PLMs could be clinically significant – particularly when often associated with arousals.
Arousals
Arousal – an abrupt shift of EEG frequency including alpha, theta or frequency higher than 16 Hz, but not sleep spindles, after at least 10 seconds of stable sleep that lasted at least 3 seconds during any sleep stage but not long enough to be classified as awake. An arousal during REM sleep is usually scored only if it is accompanied by increased amplitude of submentalis EMG.
Periodic limb movement arousal (sometimes simply called “movement arousal”) (PLMA) – PLM associated with an arousal.
Periodic limb movement arousal index (PLMAI) – number of PLMs with arousals per hour averaged over total sleep time.
Spontaneous arousal (SA) (sometimes called an arousal for no apparent reason or AFNAR) – an arousal not related to disordered breathing or movements.
Spontaneous arousal index (SAI) (also called arousal for no apparent reason index or AFNARI) – number of spontaneous arousals per hour averaged over total sleep time.
Total arousal index (TAI) – total number of arousals (related to disordered breathing plus PLMS plus spontaneous) per hour averaged over total sleep time.
Note: a generally accepted cut-off for normal vs. abnormal TAI is 5, meaning that a TAI ≤ 5 is considered within normal limits. In other words, experiencing up to 40 arousals in a typical 8 hours of sleep per night would still be considered within normal limits. Many find this observation surprising, but this underscores the worthiness of counseling patients that awakening several times per night may not be abnormal if there are no other sleep-related complaints. Yet if there are complaints such as insomnia, hypersomnia, etc., then further evaluation is likely needed.
REM sleep (Stage R) phenomena
REM sleep without atonia (RSWA) – presence of unequivocal increase in muscle tone during REM sleep and if no epileptiform discharges were noted on the record. The presence of RSWA on PSG in a patient with a clinical history of repeated dream enactment behavior confirms the diagnosis of RBD. Note: in clinical practice, RSWA is the usual finding on PSG when patients with suspected RBD undergo PSG.
Apparent dream enactment behavior (DEB) during REM sleep – simple or complex motor behavior, such as punching, kicking, jumping out of bed, spiking a football, etc., during REM sleep. This can only be ascertained by the PSG interpreter when video synchronized to PSG monitoring is viewed. Note: in clinical practice, while this finding emphatically confirms the diagnosis of RBD, the presence of apparent DEB during REM sleep is actually relatively uncommon. Furthermore, current criteria for the diagnosis of RBD are satisfied if a patient does NOT have a clinical history of repeated DEB, but does demonstrate apparent DEB during REM sleep during PSG.
Multiple sleep latency test
The multiple sleep latency test (MSLT) is the primary test used in clinical sleep medicine to objectively measure daytime hypersomnia. More specifically, the MSLT measures a patient’s ability to fall asleep, and is used primarily in the diagnosis of narcolepsy, idiopathic hypersomnia, and hypersomnia associated with an underlying neurologic disorder. This test involves the same monitoring equipment used in a PSG, except that since measuring sleep-disordered breathing parameters is not the focus of interest, those devices are not used and only the two EOG derivations, three EEG derivations, the submentalis EMG lead, and ECG are used.
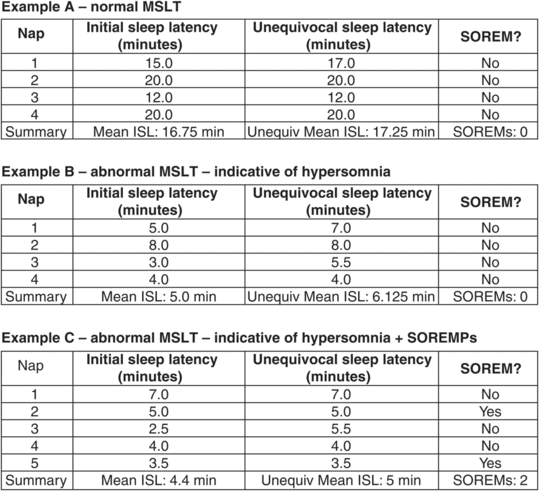
The standard “20 minute nap MSLT” is comprised of four or five nap-times with 2 hours in between each nap opportunity. Most patients complete a PSG the night prior to an MSLT to document sufficient sleep, and the PSG is usually terminated around 6–7 am. Therefore, for most patients who undergo an MSLT, the naps begin around 9 am, 11 am, 1 pm, 3 pm, and if a fifth nap is required, then this is around 5 pm. A patient is asked to lie comfortably with lights out and advised to try to fall asleep. Sleep onset is noted to occur when there are either three complete epochs of stage N1 sleep or any one epoch of unequivocal sleep. Once either sleep criterion is observed, the initial sleep latency is recorded, the patient is awoken and that nap session is terminated. If REM sleep is attained, this is noted as a “sleep onset REM” period or SOREM (this is sometimes abbreviated as SOREMP to include the “P” in period). If there is no sleep onset within a 20-minute period, then the nap session is terminated. Most MSLTs involve four naps; if a patient has one SOREM over naps one to four, a fifth nap is then performed (see below).
Since there are so many causes of abnormal findings on an MSLT (e.g., insufficient sleep syndrome, medication effect, untreated OSA, to name a few), it is critical that an MSLT be performed with all psychoactive medications tapered off weeks in advance of the MSLT (if this can be done safely), the patient has had adequate sleep every night over the week preceding the MSLT (i.e., at least 7 hours per night on average documented by sleep log or more preferably by wrist actigraphy), urine drug screen is negative, and a PSG performed the night preceding an MSLT documents at least 7 hours of total sleep time and no clinically significant sleep disordered breathing nor elevated arousals.
The primary results of interest from the MSLT are the mean initial sleep latency (mean ISL) and the number of SOREMs. Generally speaking, mean ISL values > 10 minutes are considered normal, values < 5 minutes are considered definitely abnormal, and values in the 5–10 minute range represent the “gray zone” and require clinical correlation. In those who have two or more SOREMs and a mean ISL < 5 minutes, and no other explanation for their daytime hypersomnia based on clinical history, laboratory assessment, and PSG/MSLT findings, then a diagnosis of narcolepsy may be appropriate in the correct clinical setting. In those who have a mean ISL < 5 minutes and 0 or 1 SOREM, then a diagnosis of idiopathic hypersomnia may be appropriate. Examples of normal and abnormal MSLT findings are shown in Figure 27.3.