Fig. 11.1
Schematic representation of the primary CRSWDs
Circadian rhythms are endogenous and genetically regulated and persist in the absence of external time cues with a period of approximately 24 h. A central pacemaker located in the paired suprachiasmatic nuclei (SCN) of the anterior hypothalamus regulates and coordinates the timing of nearly all physiological processes. Information from the suprachiasmatic nucleus is sent to other areas of the brain and peripheral organs to synchronize the function of the peripheral oscillators located in these tissues. In addition to the sleep–wake cycle, the SCN regulates and coordinates nearly all physiologic and behavioral processes. Therefore, when there is a disruption of the circadian system and/or misalignment between the timing of the endogenous rhythm and external environment, not only are sleep disturbances apparent, but metabolic, cardiovascular, immunological, hormonal, gastrointestinal, cognitive functions can be impaired [2].
The light–dark cycle is the strongest synchronizing agent for the circadian system. The SCN receives afferent light input primarily from the melanopsin containing retinal ganglion cells of the retina via the retino-hypothalamic tract [3, 4]. In humans, light exposure prior to the core body temperature minimum (evening) produces delays, while light pulses after the core body temperature minimum (early morning) produce advances [5]. In addition to light, the SCN also receives internal signals from the pineal gland, via the nocturnal release of melatonin. Endogenous melatonin release begins to rise 2–3 h before sleep onset and peaks in the middle of the night. Opposite to the effects of light, melatonin during the early morning induces delays in circadian rhythms, while melatonin during the early evening induces advances [6, 7]. Normal release of melatonin involves a projection from the hypothalamus, through the cervical spinal cord, and back to the pineal. Through timed activation by these synchronizing or entraining agents, the circadian clock can make daily adjustments to maintain synchronization (entrainment) with the external light–dark cycle and social and work schedules.
Circadian Rhythm Sleep–Wake Disorders
General Evaluation
Evaluation of patients with suspected CRSWD requires a thorough sleep and medical history. Evaluation of sleep–wake patterns with sleep diary and actigraphy monitoring is recommended for all CRSWDs for at least 1–2 weeks. Longer monitoring may be helpful when the sleep–wake rhythm is unstable. In addition, in patients in which the diagnosis is unclear or who have extreme sleep and wake times, more accurate biomarkers of circadian timing, such as the rhythm of endogenous melatonin, are recommended when possible.
Melatonin can be measured via plasma, saliva, or urine, though commercially available salivary kits are the most practical method in the clinical setting [8]. Because bright light exposure can suppress melatonin secretion, sampling has to be done in dim light or dark conditions starting 5–6 h before habitual sleep onset time. The timing of dim light melatonin onset (DLMO) is an excellent marker for the timing of circadian rhythms. There are different methods for determining DLMO. The most common are fixed thresholds of 3 pg/mL in salivary samples or 10 pg/mL in serum samples. Using a relative threshold that is two standard deviations above the baseline may be more accurate as baseline melatonin levels can vary from one person to another [9]. Figure 11.2 shows a general approach to the evaluation of patients with CRSWDs.
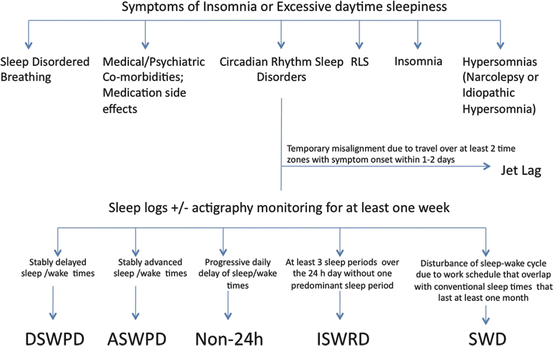

Fig. 11.2
Symptoms of insomnia or excessive daytime sleepiness
General Treatment Approach
The overall goal of treatment of CRSWD is to improve sleep quality, daytime performance, health, and safety of patients by utilizing a multimodal program that involves behavioral, environmental, and pharmacologic approaches aimed to synchronize the endogenous rhythm with that of the external environment. All patients should be educated on good sleep hygiene practices, including structuring sleep and wake times and maintaining a dark sleep environment. In addition, timed exposure to light and dark, as well as administration of exogenous melatonin, can reset the timing of circadian rhythms. It is important to keep in mind that depending on the time of exposure, these agents can advance or delay the phase of circadian rhythms, for example, melatonin given in the late afternoon to early evening (5–6 h before DLMO will advance circadian rhythms [7, 10, 11]). In contrast to melatonin, exposure to bright light in the evening (prior to DLMO) will delay circadian rhythms, and exposure in the morning will advance the rhythm. Table 11.1 shows a summary of the treatment approaches for CRSWDs.
Table 11.1
Circadian rhythm sleep disorders and their treatments
Circadian rhythm sleep disorder | Treatment |
|---|---|
Delayed sleep–wake phase disorder (DSWPD) | • Oral melatonin dosed 5–7 h prior to habitual bed time |
• Bright light therapy for 1–2 h prior to habitual wake time | |
Advanced sleep–wake phase disorder (ASWPD) | • Bright light therapy in the evening for 2 h |
Non-24 h sleep–wake rhythm disorder | • Blind: oral melatonin 1 h prior to bed time |
• Sighted: morning bright light therapy in addition to evening melatonin | |
Irregular sleep–wake rhythm disorder (ISWRD) | • Increase daytime light and activity |
• Avoid nighttime light | |
• Children: oral melatonin in the evening | |
• Elderly: combination of evening melatonin and morning bright light therapy | |
Shift work disorder (SWD) | • Naps before night shift or during shift if possible |
• Bright light, intermittent caffeine, modafinil/armodafinil at the beginning of the shift | |
• Avoid bright light at the end of the shift and during the commute home | |
• Melatonin in the morning and/or hypnotics prior to sleep time | |
Jet lag disorder | • Eastward travel: avoid bright light in the evening, bright light exposure in the mid to late morning; oral melatonin in the evening |
• Westward travel: bright light exposure in the early evening, avoid bright light in the morning |
Case 1
A 20-year-old female college student has nearly daily difficulty falling asleep for the past 2 years, but has become more pronounced about 6 months ago. She reports that most nights she goes to bed by midnight, but lies in bed for 2–4 h before falling asleep. Once asleep, however, she sleeps uninterrupted until her alarm goes off for class at 8 am. She hits the snooze button several times as she has significant difficulty getting up this early. She is very sleepy and often dozes on and off during her first two classes. She is struggling in school and has had to drop out of two classes last semester. On weekends and when she visited her parents during winter break, she would go to bed about 2 am and would generally be asleep by 3 am and would wake up between 10 am–noon. Although she felt a lot better, she was unproductive. She had tried melatonin which only sometimes helped her fall asleep.
Delayed Sleep–Wake Phase Disorder
Definition
Delayed sleep–wake phase disorder (DSWPD) is an alteration in the circadian rhythm in which the phase of the major sleep period is delayed in relation to the required sleep and wake times. This is manifested by a chronic or recurrent complaint of inability to fall asleep at a desired conventional clock time together with the inability to awaken at a desired and socially acceptable time for at least 3 months. When able to choose their preferred schedule, patients generally exhibit normal sleep quality and duration and maintain stably delayed sleep and wake times. Lastly the sleep disturbance is not better explained by another sleep disorder, medical or neurological disorder, mental disorder, medication use, or substance use disorder [1]. In addition to alterations in sleep timing, comorbidities including anxiety and depression, increased substance use, and lower grades in school have been reported [12].
Prevalence
Pathophysiology
Although the underlying pathophysiology has not been clearly elucidated, it is likely due to be multiple mechanisms. Genetics have been shown to be important. Approximately 40 % of patients with DSWPD have a positive family history, and polymorphisms in the hPer3 gene, involved in control of the circadian rhythm, have been associated with a delayed sleep phase [14]. Alterations in response to light, resulting in increased sensitivity during the evening or a decreased sensitivity in the morning, can result in a chronic delay in the phase of circadian rhythms. Most importantly behaviors may exacerbate these changes as an individual with DSWPD will likely have increased exposure to light at night as they remain awake later and low levels of morning light as their wake time is delayed [12, 15, 16]. These self-selected light and dark cycles can exacerbate and help perpetuate the delayed sleep phase.
The sleep architecture of DSWPD subjects who sleep at their preferred times is thought to be normal for age. However, the delayed phase alters the timing of both REM sleep and slow wave sleep, making it more difficult to awaken for early morning commitments [12].
Diagnosis
Diagnosis is made based primarily on a careful clinical history of chronic inability to fall asleep at night and difficulty waking up in the morning that interferes with daytime functioning. In addition, documentation of a delayed sleep–wake rhythm by at least 7 days (preferably for 14 days) of sleep log and actigraphy monitoring (if available) is required. Questionnaires can also be helpful to delineate an individual’s circadian chronotype. Patients with DSWPD are typically evening types. If the sleep log or actigraphy does not clearly document a delayed pattern, other biomarkers, such as salivary melatonin, can be collected for more objective data to confirm the delayed circadian phase.
Treatment
Treatment should be individualized to the patient depending on the magnitude of the phase delay as well as the timing of morning and early evening responsibilities. First-line therapy includes improving sleep hygiene, adherence to a structured sleep and wake schedule (including on nonwork days), avoiding bright light in the evening, and increasing light exposure in the morning [17]. However, behavioral and environmental manipulations may be insufficient for many patients with DSWPD. Due to the comorbidity with depression and anxiety disorders, it is important to consider the contribution of mood disorders in the treatment plan.
The first documented successful specific treatment was chronotherapy. This entailed delaying sleep by 3 h nightly until the patient reached the goal bedtime. Although this is efficacious, it is difficult to maintain. More practical treatments include a combination of timed bright light therapy and oral low-dose melatonin intake. Bright light exposure shortly upon awakening in the morning can help advance the timing of sleep and wake cycles, whereas exogenous melatonin in the evening has been shown to advance sleep onset time and wake time. Timing of the melatonin dose is important in influencing the effect size [10]. For a larger advance, the dose should be taken about 5–6 h prior to DLMO or 7 h before sleep onset time [7, 18, 19]. For example, in the case presented, the patient falls asleep between 2 and 3 am and wakes up naturally between 10 am and noon. Light exposure should begin at approximately 9 am, and then the timing can be advanced gradually over 1–2 weeks until the desired wake up time is achieved. If melatonin treatment is chosen for this patient, low dose between 0.5 and 3 mg should be administered at approximately 8 pm. Combined therapy with bright light and melatonin may be more effective than alone and also reduce subjective sleepiness, fatigue, and cognitive dysfunction over time [17].
Case 2
A 66-year-old female presents to her physician for chronic early morning awakening that is now significantly reducing her sleep duration and leading to daytime impairment. She continues to teach and has to be at school from 7:30 am until 4 pm. She needs to wake up by 6 am to have time to prepare, but for the past 7 months, she wakes up naturally between 3 and 4 am. She will try and lie in bed and go back to sleep. Most of the time, she will just get up after an hour of trying to fall asleep or if able to fall back to sleep, sleep is very light. She is getting on an average week about 4–5 h of sleep at night. In the evenings, she is exhausted and can probably fall asleep after dinner (7 pm), but forces herself to stay up to spend time with her husband until about 10:30 pm. She has no difficulties falling asleep. On nonwork days, she takes a nap at 1 pm for about an hour. She denies snoring or gasping arousals, witnessed apneas, discomfort in her legs, or any abnormal behaviors in sleep. She does not endorse any symptoms of depression.
Advanced Sleep–Wake Phase Disorder
Definition
In contrast to DSWPD, patients with advanced sleep–wake phase disorder (ASWPD) demonstrate a stable sleep schedule that is several hours earlier than desired sleep time and interferes with their daytime responsibilities. Sleep disturbances include difficulty staying awake until the desired bedtime and difficulty maintaining sleep until desired wake time for at least 3 months. Similar to individuals with DSWPD, when able to sleep during the preferred sleep times, sleep quality and duration are typically normal [1].
Prevalence
ASWPD is less common than DSWPD, but may be underreported, as these individuals are less likely to experience occupational disruptions. Although the prevalence in the general population is low, ASWPD is more common among older adults.
Pathophysiology
A genetic basis for some familial forms of ASWPD has been identified.
These familial cases have polymorphisms in hPer2 and casein kinase 1 delta genes that shorten the circadian period [20, 21]. In addition, behavioral and environmental exposures can also result in an earlier circadian phase. For example, a person with ASWPD is awake during the early morning hours and is more likely to be exposed to early morning light, which advances the timing of the sleep and wake rhythm and perpetuates the problem.
Diagnosis
The diagnosis is primarily based on a clinical history of early evening sleepiness and early morning awakening that is associated with impairment in functioning. However, if individuals are allowed to sleep during their preferred schedule, the sleep quality and duration are normal for age, but with a stable advanced phase. In addition, current diagnostic criteria require demonstrating an advance in the timing of the major sleep period in relation to the desired sleep and wake times. Either sleep logs alone or in conjunction with actigraphy monitoring when possible for at least 7 days (preferably 14 days) are recommended to confirm the diagnosis. The monitoring demonstrates a stable advanced sleep pattern, usually with individual sleep onset time between 6 and 9 pm and rise time between 2 and 5 am. Measurement of the DLMO can also be used to confirm the diagnosis [1].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




