and Róbert Bódizs2
(1)
Institute of Experimental Medicine and Institute of Neuroscience, Budapest, Hungary
(2)
Semmelweis University Institute of Behavioral Science, Budapest, Hungary
Abstract
During the last 15–20 years, a new knowledge accumulated about NREM slow wave oscillations that have become the key issue of homeostatic regulation. A frequency-based classification of slow waves has been developed, differentiating between 0.1–1- and 1–4-Hz groups. The cortical <1-Hz slow activity during the so-called up states (surface-positive half wave), even ripple-like (50–200 Hz) fast activity, and down state (surface-negative half wave), an interruption of synaptic and neural activity, have been described. The alternation of these two microstates ensures a unique double working mode for the cortex, providing continuity for the contact and information processing with the environment during the up states even in deep sleep and providing a separation for trophotropic functions for further cognitive demands during the down states.
With progressive development of neuroimaging, source modeling studies on sleep slow waves by new neuroimaging tools have confirmed that the cortical areas are differentially involved in slow wave production and showed that sleep slow waves can be locally – mainly frontally – regulated. They are traveling along an anterior-posterior axis largely mediated by the so-called cingulate highway. Studies in this field emphasized that those areas with maximal involvement in slow waves’ production also show considerable overlap with the default network, paradoxically implicated in monitoring the external environment, and can be altered by sleep deprivation.
Ontogenetic studies revealed that the delta oscillation associated with rapid spindling is the agent of plastic changes of the cortex. Reactive (input-dependent) delta activity seems to be an essential element of plastic changes as early as during the neonatal development. Before the fetal brain might receive elaborated sensory inputs from the external word, spontaneous fetal movements provide sensory stimulation and drive delta-brush oscillation, contributing to the formation of cortical body maps.
The spectral power of sleep slow wave activity and the steepness of the slopes of sleep slow waves were shown to correlate positively with the gray matter volume of several cortical areas in children and adolescents between 8 and 19 years of age. When the production of cortical synapses is more efficient than their elimination (from birth until the prepubertal age), slow wave activity is high and increasing; while in adulthood, when the elimination of synapses exceeds their production, the amount of sleep slow wave activity decreases.
Discussing phylogenetic relations of slow wave activity during different vigilance states and state-dependent reactions to sensory inputs, we try to interpret some paradoxical observations on reptiles. We are proposing that the reason why reptiles are in a continuous NREM sleep like condition during behavioral waking state is the lack or underdevelopment of their cholinergic arousal system. Therefore, sensory stimulation elicits K-complex-like slow wave responses. In the waking state, reptiles apparently have sleep EEG and sleep-like EEG activity during behavioral activation. Our proposal incorporates an explanation for the lack of long-term homeostatic sleep regulation in reptiles, having at the same time short-term homeostatic slow wave supplementation in response to sensory stimulation.
Keywords
Slow oscillationDelta activityInfraslow oscillationUp statesDown statesNeuroimagingOntogenesis of sleepBrain developmentPhylogenesis of sleepReptilian wakefulness7.1 A Short History of Slow Waves
The main focus of our present work is the phenomenon of slow waves, considered as a quintessence of sleep. Slow waves are also known as delta waves according to the classical EEG terminology, originating from William Gray Walter (1910–1977) in the 1930s (Walter 1936). He has performed their topographic mapping and recognized the link between brain lesions and local delta activity (meaning disease, degeneration, and death). The strong link between slow waves and sleep was first reported, also in the 1930s, by Alfred Lee Loomis (1935). He managed to make all-night EEG records of human sleep in his laboratory. This was an unbelievable technical achievement at those times as even Harvard researchers were unable to perform such records. Loomis characterized the EEG stages of sleep: the stage characterized by slow waves was called random, as the waves were considered as such in his view. He also described the increase in the amplitude of delta waves during the course of wake-sleep transition until reaching deep sleep, and most importantly he also recognized the reactivity of the slow waves and made a correct description on K-complexes evoked by knocking on the sleepers’ door (K-knock).
Thus, slow waves were considered mainly as signs of low arousal level (Loomis et al. 1935) or brain lesion (Walter 1936). This idea was present in the work of Giuseppe Moruzzi (1910–1986) and Horace Winchell Magoun (1907–1991) also who described the ascending reticular activating system in the brainstem and characterized it as follows: “The evidence given above points to the presence in the brain stem of a system of ascending reticular relays whose direct stimulation activates or desynchronizes the EEG, replacing high-voltage slow waves with low-voltage fast activity. This effect is exerted generally upon the cortex and is mediated, in part, at least, by the diffuse thalamic projection system” (Moruzzi and Magoun 1949). High-voltage slow waves were already considered as signs of sleep or low arousal in these works. This kind of “negative” view on slow waves (i.e., being signs of lack of something) persisted and was even more emphasized during and after the discovery of REM sleep in humans (Aserinsky and Kleitman 1953) or paradoxical sleep in cats (Jouvet et al. 1959). It was the appearance of fast waves instead of slow ones during sleep that seemed interesting in those times.
However, scientific interest has changed during the next few years. The search for the molecular sleep factor (S-factor) by the Pappenheimer group stimulated the search for some reliable physiological measure of sleep deprivation-induced sleep rebound. It was recognized that the best measure is sleep EEG slow wave activity. They wanted to introduce into these studies the time spent in those sleep phases characterized by EEG slow waves as well as the increase in their amplitude, instead of focusing on low-voltage fast activity (Pappenheimer et al. 1975). Similar approaches characterized the research on humoral sleep regulation (Schoenenberger et al. 1977).
Although the homeostatic regulation of slow wave sleep was already known in those studies, it was Wilse B. Webb who explicitly stated that the amount of stage 4 sleep was related to the amount of pre-sleep wakefulness (Webb and Agnew 1971). Hints on the potential recuperative role of slow wave sleep together with its precise homeostatic regulation changed the view of sleep researchers and have led to the generally accepted theories: slow waves are not merely passive expressions of reduced cortical activity due to brain lesions or low arousal; rather, they represent active self-organizational forms of neuronal activity, serving recuperative and off-line information processing functions.
7.2 Is There a Frequency-Based Typology of Slow Waves?
7.2.1 The Controversy of Below or Above 1 Hz
Mircea Steriade and his co-workers published a series of papers (Steriade et al. 1993a, b, c) reporting that:
1.
Cellular processes contributing to delta waves (<4 Hz) are inhomogeneous.
2.
The 0.1–1-Hz component of the delta (<4 Hz) activity has distinct cellular substrates and is of utmost importance in sleep rhythm generation.
3.
The 0.1–1-Hz waves are reflections of large-scale, rhythmic hyperpolarizations followed by widespread depolarization.
4.
The hyperpolarization-depolarization sequences originate from cortical neurons and are synchronized by corticocortical connections.
5.
The 0.1–1-Hz waves envelope delta and sigma (spindle) oscillations, resulting in complex, coalescent wave sequences (Fig. 7.1).
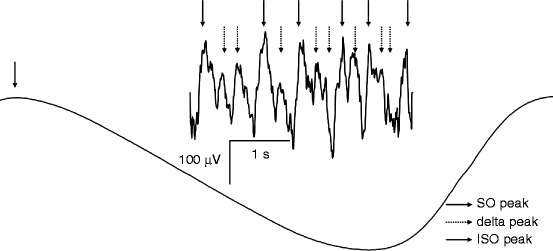

Fig. 7.1
Infraslow (0.01–0.1 Hz) oscillation (ISO), slow (<1 Hz) oscillation (SO), and delta (1–4 Hz) activity are coexisting and coalescent during slow wave sleep in humans. Negative peaks of the ISO, SO, and delta waves are shown by arrows. SO waves and delta waves emerge during the positive going phases (up states) of the ISO and SO, respectively
This novel cortical rhythm was called the slow oscillation, and the “<1-Hz” symbol was usually added in parentheses in order to make the distinction clear between the slow and the delta rhythms. According to this view, the 1–4-Hz waves are reflections of the thalamic clock-like delta activity and the cortical delta activity, while the <1-Hz component is of cortical origin reflecting different physiological processes (Amzica and Steriade 1998). A detailed scenario of sleep rhythm generation in the thalamocortical system was also given. This scenario was based on the hypothesis that the hyperpolarization-rebound sequences of thalamocortical feedback loops generating the spindle and the delta waves are triggered and grouped by the depolarization phases (up states) of the slow oscillation (Steriade et al. 1993c). The distinction between the slow oscillation and the delta waves was confirmed by the sleep records performed in animal model with the lack of T-type Ca2+ channels. The lack of this channel resulted in a significant decrease in delta and spindle oscillations, but not the slow (<1 Hz) oscillation, which remained unaffected (Lee et al. 2004). Other molecular evidences for the differentiation between the slow oscillation and the delta waves have come from pharmaco-EEG studies of sleep. Benzodiazepine hypnotics decrease NREM sleep EEG power in the delta range (>1 Hz), but may significantly increase the slower (<1 Hz) frequency components (Trachsel et al. 1990). Moreover, only the EEG frequencies <1.5 Hz, but not the higher delta bins, are affected by noradrenaline depletion in sleep-deprived rats: neurotoxic lesions with DSP-4 reduce the 0.5–1.5-Hz activity in recovery sleep, while >1.5-Hz activity remains unchanged (Cirelli et al. 2005).
Some years after the first description of the slow oscillation in animals, it was shown that its role in human sleep homeostasis is different from that of delta activity. The fast Fourier transformation (FFT) power of the <1-Hz component does not significantly decrease between the first and the second sleep cycles, while the power of the 1–4-Hz delta component does (Achermann and Borbély 1997). This was interpreted as a sign for the lack of the homeostatic regulation of the slow oscillation. The idea on missing homeostatic regulation of the slow oscillation was supported by early studies revealing a decrease in NREM sleep EEG power >1 Hz after prolonged periods of wakefulness or sleep deprivation in humans and rats (Borbély et al. 1981; Tobler and Borbély 1990). However, this conclusion was a premature one. Although the time course of delta activity is characterized by a steeper decline over the sleep cycles (NREM periods) than the time course of the slow oscillation, in physiological conditions, both seem to be under precise homeostatic regulation. This was proven by examining the effects of intervening naps in studies performed in young good sleepers (Campbell et al. 2009). Based on their different time courses, Campbell et al. (2009) attributed a permissive role to the slow oscillation in sleep homeostasis: that is, the slow waves permit the expression of the actual sleep need, measurable by the actual level of delta, embedded in sleep cycles (Fig. 7.1). The slow oscillation provides the neural and metabolic conditions for homeostatic processes. The explanation of the peculiar decrease of the <1-Hz NREM sleep EEG in recovery sleep after sleep deprivation (Borbély et al. 1981; Tobler and Borbély 1990) was given by the studies examining the frequency and the slopes of the slow waves under different levels of sleep pressure. These studies have revealed the acceleration in the alternation of up and down states, thus an increase in the frequency of the slow waves as well as an increase in the steepness of slow waves in conditions of increased sleep pressure (Riedner et al. 2007; Bersagliere and Achermann 2010; Fig. 7.2). Several results suggest that the original frequency border of <1 Hz for the slow oscillation is too narrow, and in certain conditions, frequencies higher than 1 Hz may contribute to the phenomenon. Moreover, reports of the decreased <1-Hz activity during recovery sleep find their reason in a frequency shift or acceleration of the slow oscillation. The apparent dissociation between the frequencies >1 Hz and the remaining slow wave activity may simply reflect homeostatic changes in the slopes of slow waves (Hanlon et al. 2011).
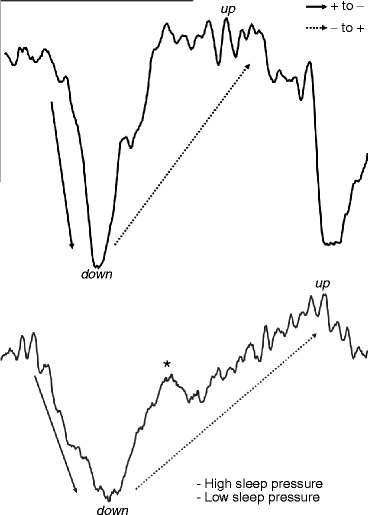

Fig. 7.2
General morphology, up and down states, as well as sleep pressure dependence of the slow (<1 Hz) oscillation in human slow wave sleep EEG. Down states are characterized by surface negativity and relative lack of superimposed higher-frequency oscillations, whereas up states are surface positive and superimposed with spindling (sigma activity). Higher sleep pressure is characterized by faster alternation of the up and the down states, steeper slopes, higher amplitude, as well as a relative lack of the well-formed multipeaks. The multipeak phenomenon during the lower sleep pressure is shown by an asterisk (*). Calibration marks: 100 μV and 1 s. EEG recordings come from stage 3 sleep of the first (high sleep pressure) and the third (low sleep pressure) sleep cycles of a young healthy subject (derivation F4-A1)
The cortical origin and corticocortical synchronization of the slow oscillation have led researchers to find the cognitive correlates of this sleep EEG rhythm. Several evidences were found for the correlation between the spectral measures of the slow oscillation and performance in different cognitive tasks. The performances were found to correlate with the sleep slow oscillation and were related to visuospatial memory as measured by the Rey-Osterrieth Complex Figure Test (Bódizs et al. 2002) and different forms of executive functions like nonverbal planning or word generation (Anderson and Horne 2003), as well as the overnight improvement in motor sequence learning (Moroni et al. 2008). Verbal learning was shown to increase EEG coherence in the up states of the slow oscillation during post-sleep learning (Mölle et al. 2004). Last, but not the least, the artificial boosting of the slow oscillation by applying transcranial direct current stimulation of 0.75-Hz frequency has significantly increased the overnight retention of paired associate verbal material (Marshall et al. 2006). It seems that there are two main cognitive features of the slow oscillation which were unraveled by the above investigations. One is a link between some type of cognitive performances and the baseline individual level of the slow oscillation. This could reflect a trait-like relationship revealing the commonalities in or reciprocal relationships between the neural background of wakeful cognitive performances and of NREM sleep cortical slow oscillation (Bódizs et al. 2002; Anderson and Horne 2003). The other type of cognitive aspect of the sleep slow oscillation is related to the sleep-dependent mechanisms of memory consolidation. There is strong evidence for the involvement of the sleep slow oscillation in the consolidation of memory traces (Marshall et al. 2006; Moroni et al. 2008).
7.2.2 Below 0.1 Hz: Infraslow Oscillation in Light of the Full-Band EEG Recordings
Direct current (DC)-EEG recordings of human sleep revealed a prominent infraslow activity roughly associated with 0.01–0.1-Hz frequency fluctuations (Vanhatalo et al. 2004; Monto et al. 2008; Picchioni et al. 2011; Hughes et al. 2011). Although the exact frequency limits of the infraslow oscillation are arbitrary and vary from study-to-study, the main issue unraveled was the evidence showing that it modulates neural excitability and interictal epileptic activity during sleep (Vanhatalo et al. 2004). Frequencies higher than 0.5 Hz were shown to be phase-coupled with (grouped by) the infraslow oscillation during both wakefulness (Monto et al. 2008) and NREM sleep (Vanhatalo et al. 2004; Fig. 7.1). The prominent very slow oscillatory patterns of preterm infants disclosed by DC-EEG during sleep were shown to take the form of long-lasting (1–5 s) occipital negative transients (200–700 μV), embedding the typical delta bursts seen in the conventional EEG (Vanhatalo et al. 2002). The power of the infraslow oscillation during sleep correlates positively with the BOLD signal intensity measured by fMRI in many subcortical regions (cerebellum, thalamus, basal ganglia) as well as some lateral cortical areas and the hippocampi. In contrast, the BOLD signal intensity of the paramedian heteromodal cortices correlated negatively with the power of infraslow activity (Picchioni et al. 2011). This was interpreted as the organization of the broad dissociation of activity between cortical and subcortical regions proven by the infraslow EEG oscillation during sleep. In vivo and in vitro data suggest that the thalamus could be a source of the infraslow oscillation (Lörincz et al. 2009; Hughes et al. 2011).
A particularly wide range of neurobehavioral phenomena fluctuate at frequencies corresponding roughly to the 0.01–0.1-Hz range. These fluctuations of autonomic nervous system activity (Lambertz and Langhorst 1998) had remained largely unmentioned in previous publications. Accumulating evidence suggests that processes providing the infraslow oscillation are the cellular substrates of some large-scale fluctuations which can appear as the CAP phenomenon in some parts of sleep.
We believe that the periodicity of the micro-arousals embedded in the CAP sequences is in fact due to the infraslow oscillation, shaping gross measures of neuronal excitability and being present in many neurobehavioral phenomena analyzed at this time scale (Table 7.1). The parallelism between these infraslow oscillations is striking when one considers that the periodic fluctuations in arousal (CAP) probably reflect the periodic fluctuations in neural excitability (infraslow oscillation). Thus, the periodicity in arousal could be gated by the periodicity in neural excitability. CAP could be a kind of an enhancement or phase resetting of infraslow activity due to internal or external sources of arousal or other forms of sleep instability. The possible link between the infraslow activity and the CAP phenomenon was suggested by other authors earlier (Lörincz et al. 2009).
Table 7.1
Neurobehavioral phenomena with periodicities in the CAP range
Neurobehavioral phenomena | Species | State | Frequency (Hz) | Period (s) | Specific remarks/conditions | Reference(s) |
|---|---|---|---|---|---|---|
Burst suppression | Human | Coma or general anesthesia | Highly variable | Cyclic simultaneous variation of EEG patterns | Fishgold and Mathis (1959) | |
Slow wave (<4.5 Hz) EEG amplitude | Human | NREM sleep | 0.031–0.047 | 21.27–32 | Achermann and Borbély (1997) | |
Theta- and alpha-band power | Human | Wakeful resting | 0.024–0.057 | 17.54–41.6 | Novak and Lepicovska (1992) | |
Ultra-slow rhythm | Human | NREM sleep | 0.05–0.025 | 20–40 | McKeown et al. (1998) | |
Alternating amplitude segments | Rat | NREM sleep | <1 | >1 | Deporteere et al. (1991) | |
Periodic oscillations in EEG and behavior | Squirrel monkey | Wakefulness | 0.06–0.03 | 15–30 | Chair-restrained, alternation of vigilance and inattentiveness | Ehlers and Foote (1984) |
Oscillation in baseline activity of basal ganglia neurons | Rat | Wakefulness | 0.5–0.016 | 2–60 | Ruskin et al. (1999) | |
Very slow oscillatory behavior of lateral geniculate neurons | Rat | Urethane anesthesia or wakefulness | 0.01–0.025 | 40–100 | Oscillation blocked by illumination of the eyes, re-induced by (non-)NMDA-antagonists or GABA agonists | Albrecht et al. (1998) |
Alternating network excitability of the hippocampus (CA1 region) | Rat | Urethane anesthesia | 0.025 | 40 | Triggers epileptic-like afterdischarges during periods of enhanced excitability | Pentonnen et al. (1999) |
Cyclic activations in the brainstem, respiration, cardiovascular system, and EEG | Dog | Chloralose-urethane anesthesia | 0.05–0.5 | 2–20 | The rhythms mutually influence their frequencies | Lambertz and Langhorst (1998) |
Infraslow EEG oscillation | Human | NREM sleep | 0.02–0.2 | 5–50 | DC-EEG recorded fluctuations, grouping higher-frequency EEG rhythms | Vanhatalo et al. (2004) |
fMRI BOLD signal fluctuation | Human | Both sleep and wakefulness | <0.1 | >10 | Fluctuations identify the default mode network (DMN) | Riedner et al. (2007) |
7.3 Up and Down States of Slow Waves
We are witnessing that the hitherto very poorly understood sleep EEG graphoelements partially described earlier come step-by-step to life and fill up by meaning. They behave as an organic system with biological role, like the pieces of wood transformed to Pinocchio, a living child with full conscience in the hands of master Geppetto, in the famous tail.
We learned from the Steriade group that different rhythms (spindles, delta, and <1-Hz slow waves on one hand and fast frequencies on the other) prevail in coalescence during slow wave sleep.
Within the cortical <1-Hz slow activity during the so-called up states (surface-positive half wave) even ripple-like (50–200 Hz) fast activity and during the “down states” (surface-negative half wave), an interruption of synaptic and neural activity alternates (Fig. 7.2). “The entire cortical network can swing rhythmically between extremely different microstates, ranging from wakefulness-like network activation to functional disconnection in the space of a few milliseconds” (Massimini et al. 2003). The “black-hole”-like down state may provide a rest phase and restoration for the most vulnerable and recuperation demanding frontal cognitive activity.
In the work of Csercsa et al. (2010) intracortical laminar local field potential gradient, multiple-unit and single-unit activities were recorded during slow wave sleep, related to simultaneous electrocorticography, and were analyzed by current source density and spectral methods. We found that slow wave activity in humans reflects a rhythmic oscillation between widespread cortical activation and silence. Cortical activation was demonstrated as increased wideband (0.3–200 Hz) spectral power including virtually all bands of cortical oscillations, increased multiple- and single-unit activities, as well as powerful inward transmembrane currents, mainly localized to the supragranular layers. Neuronal firing in the “up state” was sparse, and the average discharge rate of single cells was less than expected from animal studies. Synchronization patterns of action potentials across all cortical layers suggested that any layer could initiate firing at the onset of up states.
These findings provide strong direct experimental evidence that slow wave activity in humans is characterized by hyperpolarizing currents associated with suppressed cell firing, alternating with high levels of oscillatory synaptic/transmembrane activity associated with increased cell firing. Our results emphasize the major involvement of supragranular layers in the genesis of slow wave activity.
The recurrent cortical disfacilitation during down states together with high-level cortical activity during up states has a serious consequence for sensory transmission. The main one is that the thalamocortical sensory processing during deep slow wave sleep is not steady (Massimini et al. 2003). This could be shown by experiments where the averaging of sensory evoked potentials was grouped according to the up and down states of <1-Hz slow wave oscillation in deep sleep of animals. By this method, the amplitudes of cortical somatosensory evoked responses were found to be different: high, although not reaching wakefulness-like levels, during the up states (confirming the possibility of detecting meaningful events even in deep sleep) and low during the disfacilitation of the down state. This lack of stationarity may impair sensory integration during deep slow wave sleep.
7.4 Imaging of Slow Waves in Sleep
The activation of certain brain structures in slow wave sleep has been recorded by high-tech neuroimaging methods recently.
The advent of functional neuroimaging allowed the registration of regional cerebral blood flow (SPECT) and metabolic (PET scanning) and later neuronal activity BOLD (fMRI) changes also for sleep studies.
PET studies using/18F/-fluorodeoxyglucose as a tracer showed that global cerebral glucose metabolism in humans is lowest in stages 2–3 in NREM sleep, in contrast to REM sleep in which it is similar to the waking state (Maquet et al. 1990). Later, the resolution of PET methods substantially improved both in time and in space, and a more reliable determination of the regional differences during sleep has become possible (Braun et al. 1997; Hofle et al. 1997; Nofzinger et al. 1997; Maquet and Phillips 1998; Maquet 2000). A significant decrease (greater than in the rest of the brain) in rCBF has been shown during NREM sleep in the brainstem (dorsal pons and mesencephalon), thalamus, basal ganglia, basal forebrain, and hypothalamus and in the orbitofrontal and anterior cingulate cortex, as well as the precuneus.
The fMRI studies offer sophisticated knowledge about BOLD activation patterns related to sleep (Czisch et al. 2002, 2004, 2009; Kaufmann et al. 2006; Schabus et al. 2007; Dang-Vu et al. 2008). Several brain regions’ activities were found to be reduced during each NREM sleep stage. Most of these regions are located in the frontal lobes, but some regions of the thalamus, limbic system (anterior and posterior cingulum), as well as wider areas of the temporal, parietal, and occipital lobes, and the midbrain, hypothalamus, and mamillary bodies have also been shown to have decreased activity (Kaufmann et al. 2006). Certain patterns of activation sequences were described along the process of falling asleep, like loss of alertness during expectation of environmental stimuli as well as during deepening sleep. The decrease of insular activity gained significance related to interoceptive awareness. Special attention was paid to the crucial hypothalamic region showing globally decreased activity reflecting to the decreased firing of wake-promoting neurons. It is interesting that “some pronounced synchronous BOLD activity changes” were observed in the hypothalamus in NREM that correlated with a simultaneous activity in wider cortical areas resembling to the activity in the pathways of the ascending arousal system during NREM sleep. Kaufmann et al. (2006) emphasize the role of a hypothalamic-driven network regulation both in the initiation and maintenance of sleep. They observed a decreased activity of the hypothalamic regions throughout NREM sleep stages, reflecting the reduced activity of wake-promoting neurons. Due to limited spatial resolution, differentiating hypothalamic subregions was not possible. However, “some pronounced synchronous BOLD activity changes that correlated with the hypothalamus occurred: limbic structures, regions of the frontal and parietal cortex, the basal forebrain and brainstem showed a similar time course of activation as hypothalamic regions did.” Kaufman et al. interpreted this pattern of activation as resembling to the pathway of the ascending arousal system which sends projections from the brainstem and posterior hypothalamus throughout the forebrain. These findings are congruent with the role of arousal-driven phasic activity, as described in this book. Phasic increases were observed in regional brain activity in relation to discrete events as slow waves or delta waves, compared to baseline sleep activity (Dang-Vu et al. 2008). Delta waves (>1 Hz) were associated with frontal responses. Significant increases in activity were associated with the <1-Hz waves in several cortical areas, including the inferior frontal, medial prefrontal, and precuneus and posterior cingulate areas. The <1-Hz slow waves are associated with significant activity in the parahippocampal gyrus, cerebellum, and brainstem, whereas delta waves are related to frontal regions compared to baseline activity. No decrease of activity (negative BOLD signal) was observed. A partial overlap was seen between the default network of wakefulness and responses associated with slow wave oscillation. They unexpectedly found significant responses associated with slow oscillations in the midbrain and pontine tegmentum, especially in regions of cholinergic and aminergic nuclei. This particular finding again points to traces of arousal activity during NREM sleep, the importance of which was highlighted in Chap. 6
Using high-density EEG and source modeling methods, sleep slow waves occurring spontaneously and evoked by transcranial magnetic stimulation were studied (Murphy et al. 2009). It was found that some areas of the cortex are differentially involved in slow waves and that sleep slow waves can be locally regulated. Slow waves as a group were associated with large currents in the medial, middle, and inferior frontal gyri, the anterior cingular, the precuneus, and posterior cingular regions. This study again emphasized that areas showing maximal involvement in slow waves also show considerable overlap with the default network which is paradoxically implicated in monitoring the external environment and can be altered by sleep deprivation.
Further understanding of the role of slow wave oscillation and particularly of the up and down states in plastic changes during sleep would be crucial; however, spatial resolution of fMRI does not allow us to reveal the time and space structure of cortical up and down states during NREM sleep changes.
7.5 Relation of K-Complexes and Sleep Slow Waves
We have shown that K-complexes express a double nature (Janus-faced): they show responsivity to sensory (mainly acoustic) stimuli and behave as slow waves following the homeostatic decay increasing after sleep deprivation and being related to the deepness of the actual sleep cycle. Furthermore, the studies of Amzica and Steriade (1998) and later Cash et al. (2009) proved that the main surface-negative large N550 component of K-complexes is characterized by an important decrease of unit discharges and synaptic activity in the frontal cortex similar to the “down state” of the frontal slow oscillation during NREM sleep (Fig. 3.4).
According to the fMRI studies of Riedner et al. (2011), even the spontaneous K-complexes wear, at their initial segment, the traces of multisensory processing. Therefore, we may consider K-complexes as the most characteristic prototypes of the “reactive slow wave activity.” Although K-complexes can be well obtained by the averaging also in deep slow wave sleep (Fig. 3.3), they are more frequent in stage 2.
K-complexes are essential constituents of CAP sequences, mainly in the A1 phase events. At the same time A1 events occur much more frequently during the descending slopes of the first cycles, but both spontaneous and elicited K-complexes have higher frequency during the “A” slope. This difference in distribution is even more pronounced if we consider the behavior of evoked K-complexes under continuous random sensory stimulation (Halász 1982). While less reactive slow activity can be elicited in the form of CAP A1 phases during the “A” slopes of the first cycles, the responsivity in the form of singular K-complexes is more preserved compared to the “D” slopes.
Thus, K-complex occurs more frequently during stage 2 sleep and during “A” slopes of cycles and seems to be more linked to the arousal compared to other sleep slow waves. If we involve into the group of reactive sleep graphoelements the classical vertex sharp transients, a continuum of these graphoelements can be outlined more clearly. In superficial sleep (stage 1–2), sensory (mainly acoustic) stimuli evoke the vertex sharp wave, which can be considered as an exaggerated form of acoustic evoked potential, without any important slow wave constituent. Later, in stage 2, the stimulation evokes K-complexes instead of vertex waves, with recognizable remnants of the acoustic evoked potential, but the essential part is the slow wave complex (N350-N550-P900, see Fig. 3.3). Beginning from stage 2, in deeper NREM sleep, the evoked response becomes more complex in the form of CAP A1, which incorporates K-complexes, slow waves, and spindles, while the original evoked potential compartment is hardly visible. The response to sensory stimulation shifts from a multimodal evoked potential-type response to a nonspecific bilateral, frontally dominant slow wave response.
The recent functional neuroimaging results seem to confirm this continuum. Stern et al. (2011), investigating vertex sharp wave generation by fMRI, have shown that BOLD positive activations of regions principally encompass the primary sensorimotor cortical regions for vision, hearing, and touch. Earlier in this book, we detailed the findings of Riedner et al. (2011) who had highlighted the initial evoked potential compartments before the slow wave components of K-complexes. The fMRI counterparts of sleep slow waves were discussed in detail in Sect. 6.2.
The special situation of K-complex in sensory information workup and in fulfilling trophotropic and plastic functions of NREM sleep is nicely supported by the findings of Jahnke et al. (2012). In their fMRI study, they found that K-complex is associated with positive BOLD signal changes in subcortical (brainstem and thalamus), cerebellar, sensory (auditory, visual, and sensorimotor) motor, and midline (anterior and midcingular gyri, precuneus) areas and regions which form part of the so-called default mode network (DMN) (prefrontal cortex, inferior parietal lobule, precuneus). Negative BOLD signal changes were observed in bilateral insular cortices. The primary auditory cortex was the first cortical region to be influenced during K-complex.
These findings confirm “the central role of the thalamus in the mediation of cortically generated K-complex leading to sleep spindles and slow waves” and also that “K-complex permits a low level information processing in one hand and a sleep protective counteraction on the other.” The DMN-type activation is interpreted in line with the “sentinel hypothesis,” describing DMN activity “in the context of a broad low level focus of attention when one – like a sentinel – monitoring the external world for unexpected events.” This version of the Hamletian question (“to wake or not to wake”) is very near to our interpretation (Halász 2005).
Summing up, within the continuum of reactive sleep graphoelements, K-complex seems to be situated between the evoked potential type and reactive slow wave type responses (Table 7.2). The functional significance of this Janus-faced character is that during K-complex, the brain decides “not to wake up” and compensates the disturbing effect of the incoming stimulus by producing slow wave activity.
Table 7.2
Continuum among the reactive graphoelements in NREM sleep
Reactive sleep graphoelements | Vertex wave | K-complex | Slow waves |
|---|---|---|---|
Reactivity | Spontaneous/evoked | Spontaneous/evoked | Spontaneous/evoked (CAP A1) |
Electrophysiology | Enlarged nonspecific evoked potential | Late slow wave component of evoked potentials, cortical down state, limited connection with cognition | Frontal origin (source modeling), traveling wave, up-down state alternation, connection with homeostatic and plastic functions |
fMRI | BOLD activation in sensorimotor regions + vision, hearing, touch | Activation of auditory cortex + sensory areas, thalamic activation, DMN activation | Frontal sources |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








