Chapter 14
Specialized Techniques
14A
Multiple Sleep Latency Testing
Indications for Multiple Sleep Latency Test
The American Academy of Sleep Medicine (AASM) Standards of Practice Committee recommended indications for MSLT. Narcolepsy is the single most important indication for performing the MSLT (Fig. 14A.1). A mean sleep latency of less than 8 minutes combined with SOREMs in two or more of the four to five recordings during MSLT is strongly suggestive of narcolepsy, although REM sleep dysregulation and circadian rhythm sleep disorders may also lead to such findings.
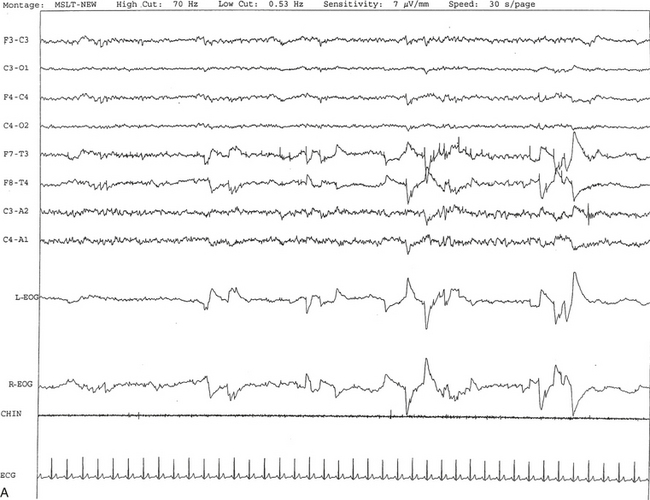
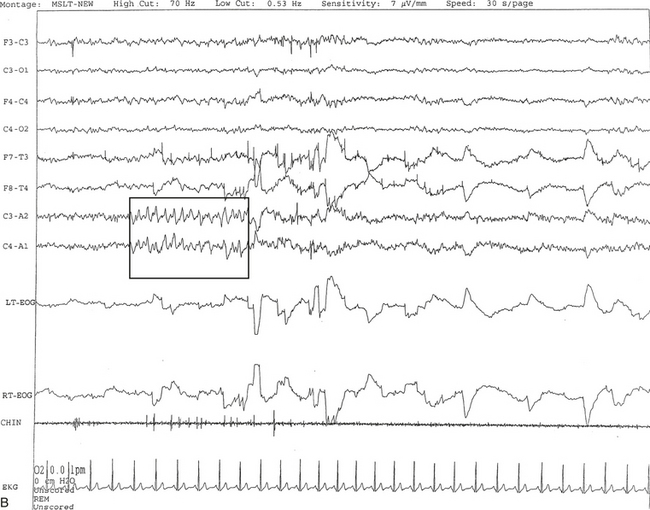
FIGURE 14A.1 A case of narcolepsy.
A 60-year-old woman with new onset of intermittent episodes of sudden transient bilateral leg weakness, excessive daytime sleepiness, and intermittent periods of transient confusion. A daytime electroencephalogram (EEG) was normal. Overnight polysomnography was significant for sleep architecture changes with an immediate sleep-onset latency, presence of only one rapid eye movement (REM) cycle, with a decreased REM sleep percentage (7%), an increased arousal index of 23 without associated apnea or periodic limb movements, and with excessive fragmentary myoclonus in non-REM and REM sleep. The multiple sleep latency test (MSLT) showed a mean sleep latency of 1.6 minutes consistent with pathological sleepiness and the presence of two (out of four) sleep-onset REM naps, suggestive of REM sleep dysregulation as seen in narcolepsy. A, A 30-second epoch from the MSLT showing the presence of sleep-onset REM occurring 7 minutes after sleep onset. Prominent REMs are seen in the electro-oculogram (EOG) channels and anterior temporal EEG electrodes. B, A 30-second epoch taken from the same sleep nap as in A, showing the presence of prominent sawtooth waves (boxed) in C3 and C4 electrode channels referenced to contralateral ears. Eye movements characteristic of REM sleep are noted as described earlier. Top eight channels, EEG; L-EOG and R-EOG, left and right EOG; CHIN, electromyography of chin; ECG, electrocardiography.
Maintenance of Wakefulness Test
The maintenance of wakefulness test (MWT) is a variant of the MSLT that measures an individual’s ability to stay awake. It should be clearly understood that the MSLT and MWT assess separate functions: The MSLT unmasks physiological sleepiness, which depends on both circadian and homeostatic factors; in contrast, the MWT is a reflection of the individual’s capability to resist sleep and is influenced by physiological sleepiness.
Indications for the MWT
The AASM Standards of Practice Committee recommended the following indications for the MWT:
• Assessment of individuals employed in occupations involving public transportation or safety for their ability to remain awake
• Assessment of response to treatment (e.g., response to stimulants in narcolepsy and CPAP titration in OSAS patients)
Aldrich, M. S. Sleep Medicine. New York: Oxford University Press; 1999.
Arand, D., Bonnet, M., Hurwitz, T., et al. The clinical use of the MSLT and MWT. Sleep. 2005; 28:123–144.
Carskadon, M. A., Dement, W. C., Mitler, M., et al. Guidelines for the multiple sleep latency test MSLT: A standard measure of sleepiness. Sleep. 1986; 9:519–524.
Chervin, R. Assessment of sleepiness. In Chokroverty S., Montagna P., Allen R. P., Walters A. S., eds. : Sleep and Movement Disorders, 2nd ed, New York: Oxford University Press, 2013.
Doghramji, K. The maintenance of wakefulness test. In: Chokroverty S., ed. Sleep Disorders Medicine: Basic Science, Technical Considerations and Clinical Aspects. 3rd ed. Philadelphia: Saunders/Elsevier; 2009:224–228.
Littner, M. R., Kushida, C., Wise, M., et al. Practice parameters for clinical use of the multiple sleep latency tests and the maintenance of wakefulness test. Sleep. 2005; 28:113–121.
14B
Actigraphy
An actigraph, also known as an actometer or actimeter, monitors body movements and other activities continuously for days, weeks, or even months. This can be worn on the wrist or alternatively on the ankle for recording arm, leg, and body movements. An actigraph uses piezoelectric sensors that function as accelerometers to record acceleration or deceleration of movements rather than the actual movement. The mechanical movements are converted into electrical signals, which are then sampled every tenth second over a predetermined time or epoch and then retrieved and analyzed in a computer. The principle of analysis is based on the fact that increased movements (as indicated by black bars in the actigraph) are seen during wakefulness in contrast to markedly decreased movements or no movements (as indicated by the white area interrupting the black bars) during sleep, although normal physiological body and limb movements and postural shifts during sleep will cause interruptions (black bars) of the white background (Fig. 14B.1). Several actigraph models are in the development stage to carefully regulate the sampling frequencies and duration, filters, sensitivities, and dynamic range to detect and quantify periodic limb movements in sleep (PLMS), but no generally accepted standardized technique of quantifying and identifying PLMS that differentiates them from other movements (e.g., those resulting from parasomnias, nocturnal seizures, and other dyskinesias) is currently available. Two of these devices (i.e., Actiwatch and PAM-RL) showed a reasonable correlation with PLMS observed with PSG study. Further validation studies in a large number of patients are needed before these can be used reliably to detect and quantify PLMS. Currently several devices are available (e.g., ambulatory monitoring, Actiwatch, PAM-RL) along with appropriate software, with or without light or position sensors, to determine if the subject is sitting, lying, or upright. These devices can provide a direct assessment of the amount of activity or body movement at the site of attachment of the device. Studies have been conducted using actigraph and PSG recordings simultaneously to validate the ability of the actigraph to distinguish sleep from wakefulness. Computer algorithms are available for automatic sleep-wake scoring; however, visual inspection of the raw data is necessary. The reliability and validity of the data are available only for a specific actigraph model; no universally validated data are available. There is poor agreement between sleep characteristics obtained by actigraphy and subjective data from a sleep diary. Actigraphs can differentiate indirectly sleep from wakefulness (see Fig. 14B.1) but cannot differentiate REM from NREM sleep and cannot identify different NREM sleep stages. Actigraphs and sleep logs are complementary.
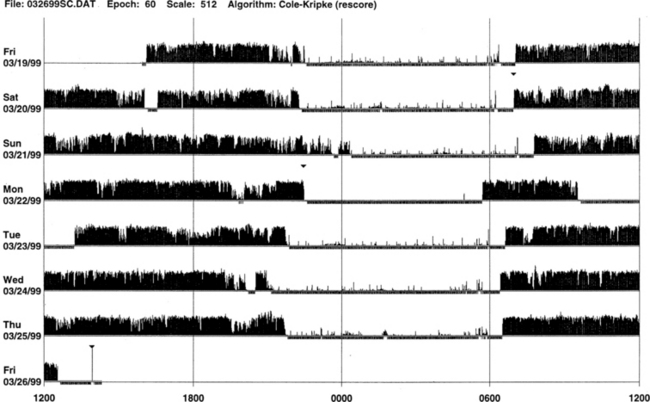
FIGURE 14B.1 Normal sleep-wake schedule.
Wrist actigraphic recording from a 55-year-old healthy woman without sleep complaints. This shows a fairly regular sleep-wake schedule except one weekend night (third from the top). She goes to bed between 10:30 PM and 11:00 PM and wakes up around 7:00 AM except on the third day. Physiological body shifts and movements during sleep are indicated by a few black bars in the white areas. The waking period is indicated by black bars.
Indications for Actigraph
The AASM Standards of Practice Committee made the following recommendations for actigraph:
• Actigraph may be a useful adjunct to history, physical examination, and sleep logs in patients with insomnia, including paradoxical sleep (sleep state misperception) and inadequate sleep hygiene (Figs. 14B.2 to 14B.7), and circadian rhythm sleep disorders (Figs. 14B.8 to 14B.13).
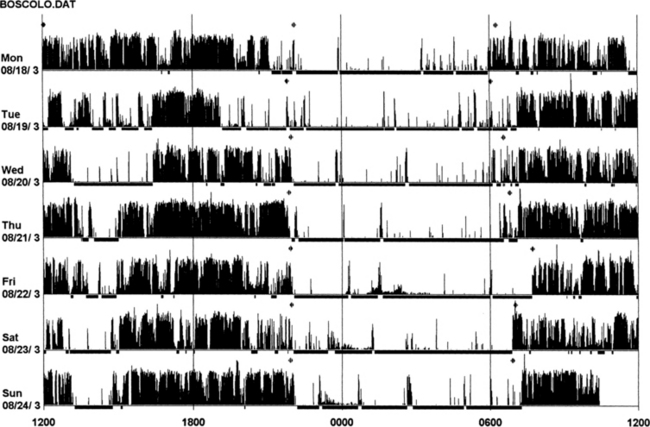
FIGURE 14B.2 Actigraphy in insomnia (sleep state misperception).
A 59-year-old man complaining of insomnia since the age of 12 years. His DSM IV axis 2 personality disorder (dependent personality) and panic attacks were diagnosed in the past and treated with benzodiazepines (chlordesmethyldiazepam 3 mg; flurazepam 30 mg) and zolpidem 10 mg. He denies any symptoms of restless legs syndrome, excessive daytime sleepiness, or daytime sleep attacks. Subjective sleep duration is 3 to 4 hours per night. In the past he had numerous drugs for sleep amelioration, but no clear and stable subjective improvement was noted. An actigraphic monitoring (during drug-reduction, chlordesmethyldiazepam 2 mg, flurazepam 15 mg, and no zolpidem) shows a clear misperception of sleep duration and quality. The recording shows normal nocturnal motor activity and sleep efficiency and duration. Note sleep period during the afternoon. He complained of sleeping not more than 3 hours each night. Polysomnography on the third night: total sleep time, 387 minutes; sleep efficiency, 73.5; wake after sleep onset, 122 minutes; number of awakenings, 17; slow wake sleep percentage, 1.3; periodic limb movements in sleep index, 1.9. DSM IV, Diagnostic and Statistical Manual of Mental Disorders, fourth edition.
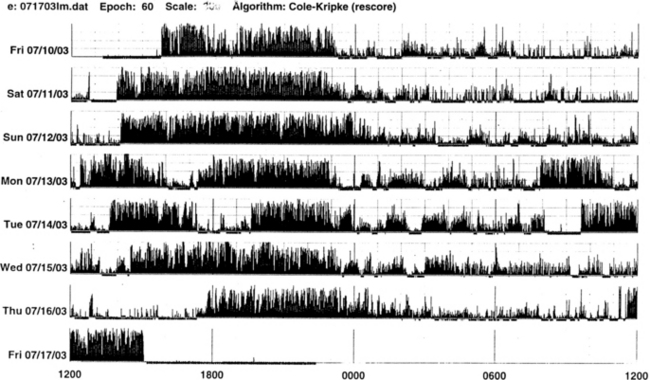
FIGURE 14B.3 A case of chronic insomnia.
Wrist actigraph from a 71-year-old woman with complaints of sleep onset and maintenance insomnia for 30 years. She has had many hypnotics over the years with temporary benefits. Despite increased doses, the effects wore off over the years. She has had many “street drugs,” and now she takes cocaine every night at bedtime. She also complains of excessive daytime somnolence. The actigraph shows many periods of wakefulness (intrusion of black bars) during sleep period (white area) and intermittent brief periods of somnolence (intrusion of white areas into the black bars) during daytime wakefulness. This is an example of hypnotic-dependent and stimulant-dependent sleep disorder.
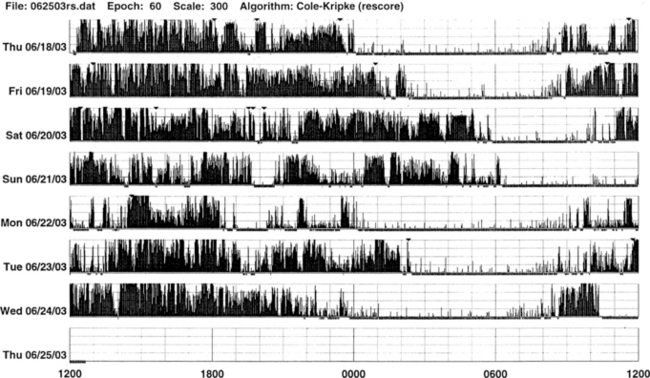
FIGURE 14B.4 A patient with sleep-onset insomnia and sleep apnea.
Wrist actigraph from a 31-year-old man who has been complaining of sleep-onset insomnia and excessive daytime sleepiness. The actigraph shows irregular sleep onset (delayed) and irregular wake-up time. There are periods of somnolence (intrusion of white areas into black bars) during waking period (black bars). Overnight polysomnographic study showed moderately severe upper airway obstructive sleep apnea (apnea-hypopnea index of 39.8) with oxygen desaturation.
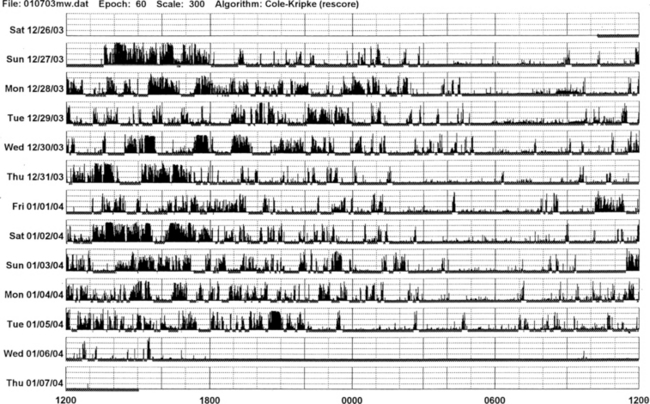
FIGURE 14B.5 Parkinson’s disease and sleep disorder (see also Fig. 12.2).
Actigraphic recording from the right ankle of a 67-year-old patient with idiopathic Parkinson’s disease (Hoehn-Yahr stage 2: mild-moderate). The patient complains of insomnia and daytime hypersomnia. The actigraph shows disorganization of sleep-wake schedule with irregular sleep-wake onset and offset, increased activity (black bars in the white area) during nighttime sleep, and many periods of daytime somnolence (intrusions of white areas within the black bars). Overnight polysomnography shows evidence of upper airway obstructive sleep apnea syndrome (apnea-hypopnea index of 25) and oxygen desaturation.
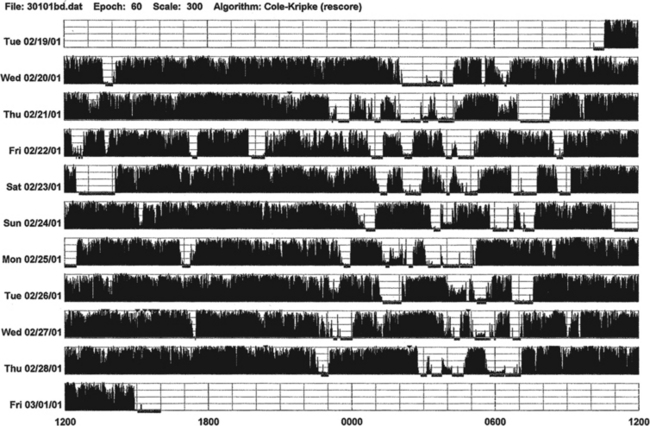
FIGURE 14B.6 A patient with advanced Parkinson’s disease and chronic sleep deprivation.
This ankle actigraph, from a 65-year-old man with advanced Parkinson’s disease (Hoehn-Yahr stage 4), shows a complete disorganization of sleep-wake cycling. Between midnight and 7:00 AM (sleep period) there are frequent short episodes of sleepiness (white areas) and wakefulness (black bars). During waking period (7:00 AM to midnight) there are episodes of sleepiness (white areas intruding into black bars).
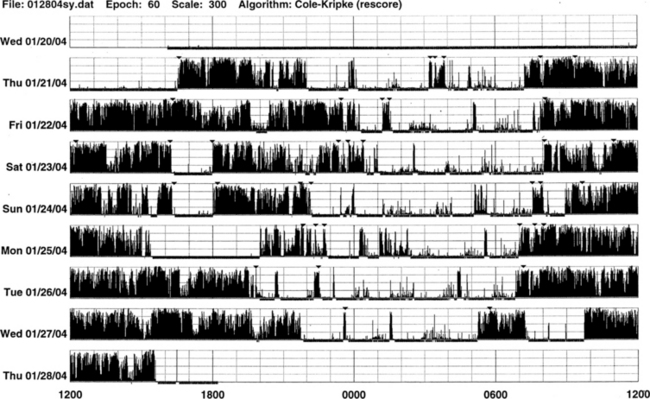
FIGURE 14B.7 Wrist actigraph from a 52-year-old psychologist with type I Arnold-Chiari malformation, who presented with sleep difficulties because of repeated awakenings and excessive daytime somnolence for 15 years (see Fig. 12.6). Overnight polysomnography showed rapid eye movement hypoventilation. The actigraph shows increased activity and periods of wakefulness during nighttime sleep (intrusions of black bars into white areas), irregular sleep onset at approximately 10:00 PM (day 1), midnight (day 2), 1:00 AM (day 3), 10:00 PM (day 4), 11:00 PM (day 5), 8:00 PM (day 6), and 10:00 PM (day 7), and variable wake-up times as indicated by sustained black bars. There are also many brief episodes of daytime somnolence (intrusions of white areas into black bars). On day 5 the actigraph was taken off during part of the waking period as indicated by abrupt onset of white area (no activity).
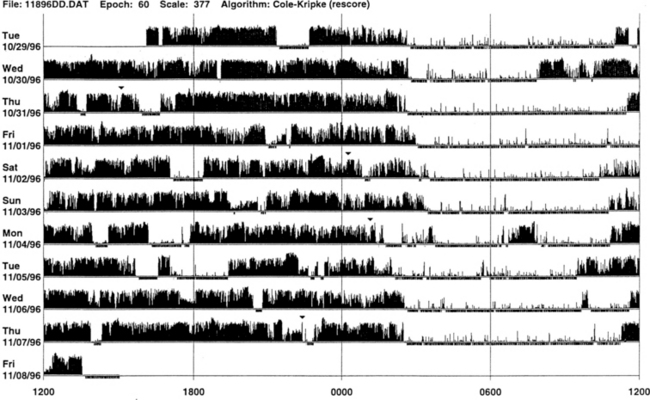
FIGURE 14B.8 Primary delayed sleep phase syndrome.
Wrist actigraphic recording from a 29-year-old man with a lifelong history of delayed sleep onset and delayed wake-up time. The actigraph shows his typical sleep period from 3:00 AM to 4:00 AM to 9:00 AM to noon (white areas). If he has to wake up early in the morning, he feels exhausted and sleepy all day. He feels fine if he is allowed to follow his own schedule. Melatonin at night did not help him. Morning bright light therapy was suggested, but the patient declined.
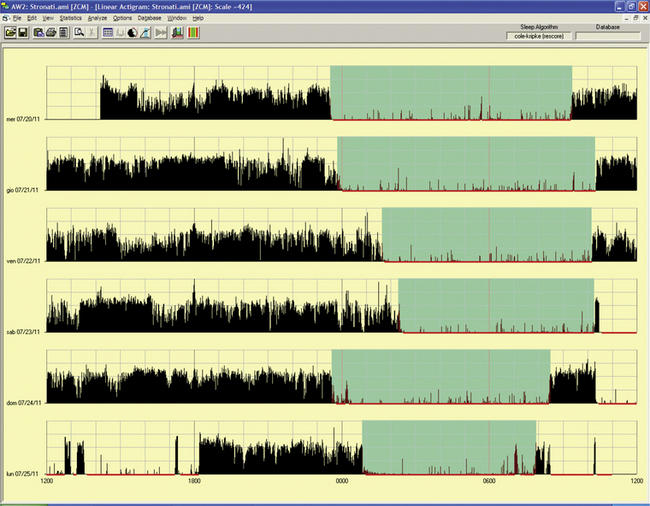
FIGURE 14B.9 Delayed sleep phase.
A 15-year-old girl complaining of difficulties falling asleep since the age of 2 to 3 years with tendency to sleep, at that time, from 11 PM to 10 AM. She complained of difficulties in falling asleep at the social time schedule for a teenager, with sleep latency of up to 2 hours with a time in bed at 11 PM and more difficulty waking up in the morning for school. Moreover, she had some problems with progress at school and sometimes experienced a “completely awake night.” Actigraphy for 6 free-running days showed a tendency to delay sleep onset (from midnight to 2 AM, more during the weekend) and awakening after 9 AM to 10 AM, excluding the last night. Estimated sleep efficiency results were very good in all of the nights (ranging from 92% to 98%).
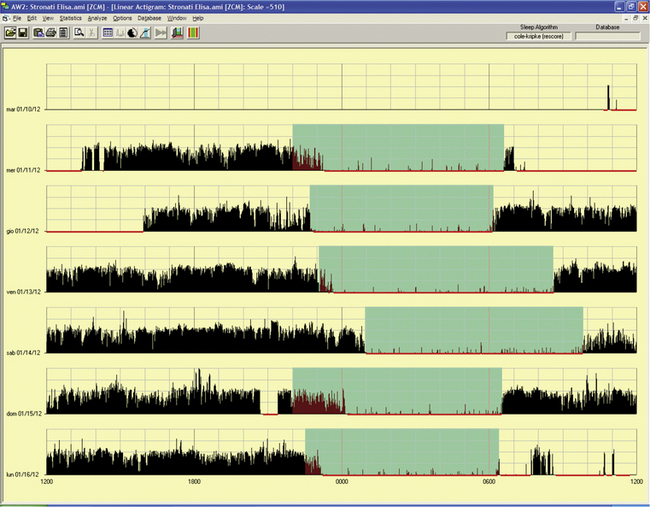
FIGURE 14B.10 Delayed sleep phase.
The same patient as in Fig. 14B.9, treated with melatonin 1 mg per night (at 8 PM). Note in a free-running 6-day period an advance of 1 to 1½ hours for sleep onset and 2 hours for awakening except for occasional delays (third and fourth nights) during the weekend that were behaviorally induced.
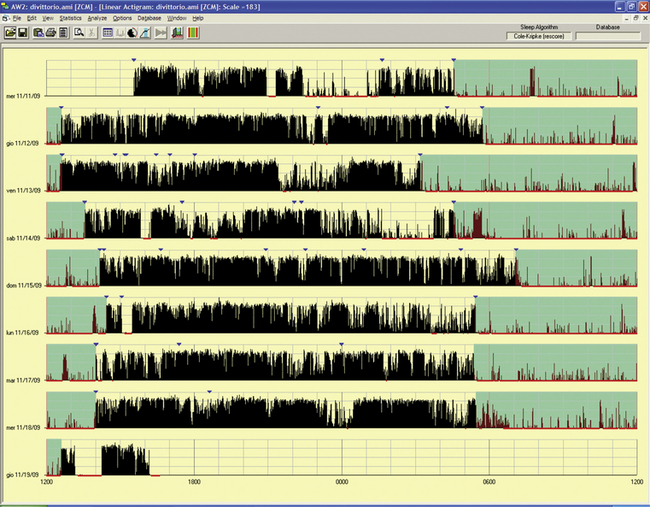
FIGURE 14B.11 Delayed sleep phase disorder.
A 55-year-old woman with a tendency to have difficulty falling asleep at night and a tendency to fall asleep during the day since childhood. She experienced progression of the disorder with age and chose a work schedule from 11 AM to 7 PM. There is a family history for evening type (brother). The sleep diary showed sleep from 4:30 AM to 5 AM until noon to 1 PM if free running. Actigraphy for eight day-night periods confirmed a delayed sleep (range 4:30 AM to 6 AM to 12.30 PM to 2 PM) with an estimated sleep efficiency above 90% in all of the nights except the last night (79.8%). Note an attempt to sleep in the evening/night on two occasions (first and fourth nights), with short duration and increased activity, indicating poor sleep quality. Treatment with melatonin in the evening and bright light in the morning was started.
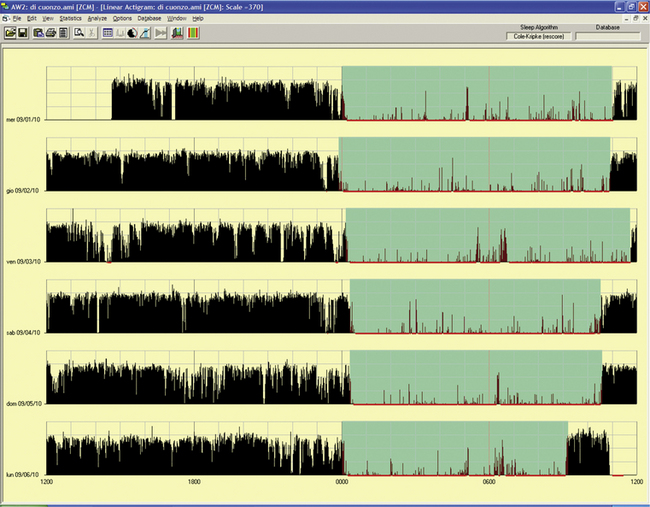
FIGURE 14B.12 Delayed sleep phase rhythm.
A 10-year-old-girl whose mother described the girl’s difficulty falling asleep before midnight and trouble awakening in the morning for school. During weekends or vacations the girl sleeps from 1 AM to noon. She reported nightmares or fearful dreaming. The father has an evening type of sleep-wake rhythm, and three siblings have normal day-night rhythms. Actigraphy showed a very stable sleep-wake schedule in a free-running 6-day period: sleep onset around midnight and awakening ranging from 10:30 AM to 11:30 AM. The last night’s recording showed a provoked awakening at 9 AM to deliver the actigraphy results.
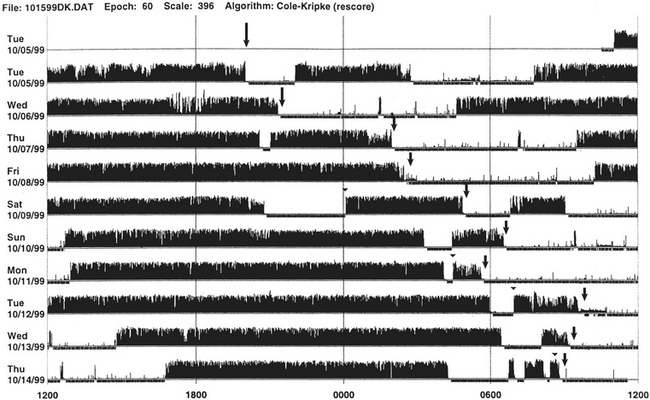
FIGURE 14B.13 Sleep-wake schedule disorder in a patient with acquired immunodeficiency syndrome (AIDS).
Wrist actigraph from a 46-year-old man with AIDS. The patient presented with sleep difficulty caused by inability to fall asleep and wake at desired bedtime and wake-up time. This 10-day recording shows disorganization of the sleep-wake schedule. There is a suggestion of non–24-hour (hypernychthemeral) syndrome with progressive delay of sleep onset (arrows) from day 1 to day 6, and again delayed sleep onset (arrows) from day 7 to day 10.
• Actigraph may be a useful adjunct to detect the rest-activity patterns during modified portable sleep apnea testing.
• Actigraph is useful to document rest-activity patterns over days and weeks when a sleep log is not able to provide such data.
Although not adequately standardized, actigraph may have a role in patients with restless legs syndrome and PLMS (Figs. 14B.14 to 14B.16). It may also be useful to document objectively periodic hypersomnias as seen in patients with Kleine-Levin syndrome (Fig. 14B.17).
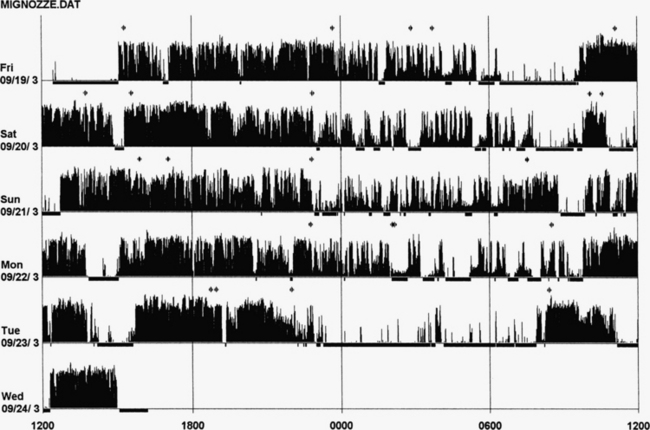
FIGURE 14B.14 Actigraphy in restless legs syndrome (RLS).
A 50-year-old woman with a 2-year history of RLS and a recent increase in severity (International Restless Legs Syndrome Study Group [IRLSSG] score of 32), of symptoms, and of difficulty in falling asleep (mean 2 hours). Her father and brother are both affected, and she is unresponsive to benzodiazepines (lorazepam, clonazepam) and zolpidem. Five nights of monitoring with actigraphy at the calf, 4 days without treatment, and the fifth with dopamine agonist (pramipexole 0.125 mg 2 hours before bedtime). Actigraph shows an increase in nocturnal leg activity during the night (probably periodic limb movements) and an increase in number of awakenings in 4 nights. The last night shows very clear increase in sleep duration and quality with a decrease in nocturnal motor activity. Polysomnography on the fourth night: total sleep time, 356 minutes; sleep efficiency, 56.1; wake after sleep onset, 224; 19 awakenings; periodic limb movements in sleep index, 82.2.
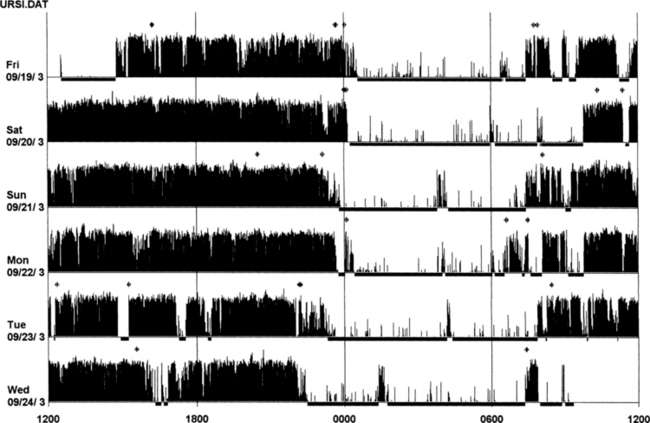
FIGURE 14B.15 Actigraphy at the calf in restless legs syndrome (RLS).
A 59-year-old woman with a 9-year history of mild to moderate RLS (International Restless Legs Syndrome Study Group [IRLSSG] score of 22), positive family history for RLS, treated with lorazepam 1 mg for several years. One week of actigraphic monitoring at the calf with an increase in nocturnal motor activity in four out of six nights and reduced sleep efficiency in the same nights. Polysomnography on the fourth night: total sleep time, 345 minutes; sleep efficiency, 92.7; wake after sleep onset, 24 minutes; number of awakenings, 7; periodic limb movements in sleep index, 4.9. Compare these findings with those in Figure 14B.14 (severe form of RLS).
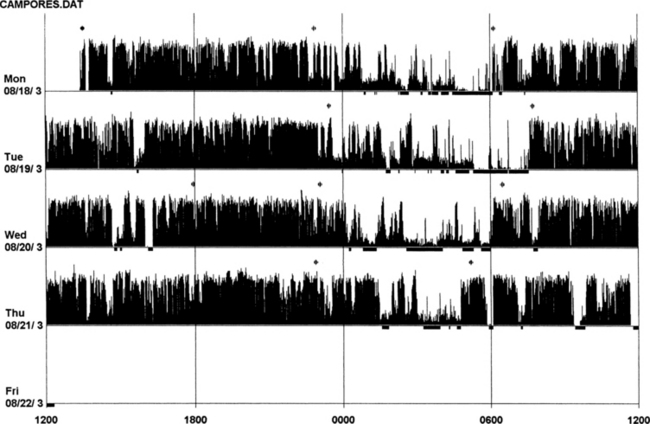
FIGURE 14B.16 Actigraphy of severe restless legs syndrome (RLS).
A 60-year-old woman with familial idiopathic RLS since the age of 30 years but increased in severity for the past 6 years. Symptoms are prominent between 8 PM and 11 PM. Sometimes RLS also occurs during the day. She has received treatment with ropinirole (slight effect), L-dopa (augmentation after 2 months), and pramipexole as an add-on to L-dopa for 2 months. She now experiences RLS during the day and night with 3 to 4 hours (maximum) of sleep per night (International Restless Legs Syndrome Study Group [IRLSSG] score of 35). Polysomnography (the second night of actigraphic monitoring, during treatment with L-dopa 250 mg and pramipexole 0.50 mg): total sleep time, 421 minutes; sleep efficiency, 81.3; wake after sleep onset, 96 minutes; number of awakenings, 19; periodic limb movements in sleep index, 108.
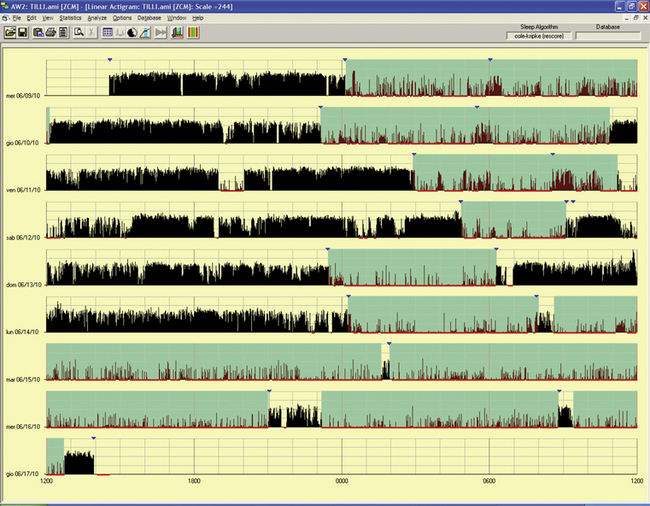
FIGURE 14B.17 Periodic hypersomnia (Kleine-Levin syndrome type).
A 29-year-old man with a history of alternate periods of insomnia and hypersomnia, with irregular sleep-wake rhythms associated with hyperphagia; this was treated as a psychiatric problem. Polysomnogram during an out-of-phase period showed no abnormalities in multiple sleep latency test (sleep latency, 13 minutes) and nocturnal sleep (only a reduced rapid eye movement sleep percentage). Actigraphy for an eight-night/day period had a chance to record a period of hypersomnia (last three lines): the patient complained of being unable to wake and stand up in the morning and of hyperphagia and some manifestations of hypersexuality during the day. Note the difference between the first three nights with a long sleep period, but with increased motor activity, and the next two nights of insomnia before going into the hypersomniac period.
Advantages of Actigraph Over Polysomnography
The advantages include the following: easy accessibility; inexpensive recording over extended periods for days, weeks, or even months; recording of 24-hour activities at all sites (home, work, or laboratories); usefulness in uncooperative and demented patients when laboratory PSG study is not possible; ability to conduct longitudinal studies during therapeutic intervention (behavioral or pharmacological treatment) in patients with insomnia; usefulness in sleep state misperception (see Fig. 14B.2); ability to document delayed or advanced sleep phase syndrome or non–24-hour circadian rhythm disorders, although sleep logs may suggest such a diagnosis; and documentation of excessive daytime sleepiness and repeated episodes of sleepiness (lasting more than a few minutes).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







