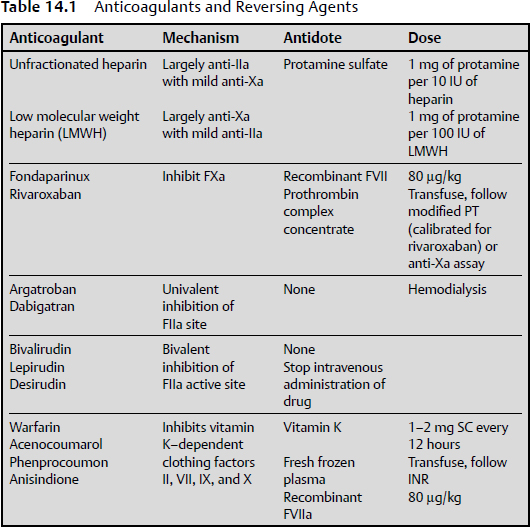
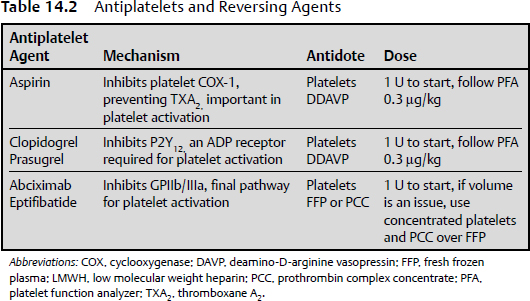
14 In 1916, a medical student named Jay Mclean stumbled across a substance that inhibited blood coagulation.1 It was not until approximately 20 years later that heparin was isolated in enough quantity to use clinically.2 Since that time, numerous anticoagulant and antiplatelet agents have been developed to treat pathological thrombosis. Pathological processes requiring anticoagulation and antiplatelet therapy in neurology may include venous sinus thrombosis, ischemic stroke, intracranial stent-assisted coiling, and carotid and vertebral artery dissection. However, many patients brought to the attention of neurosurgeons are prescribed anticoagulant and antiplatelet drugs to treat coronary and peripheral vascular disease, or are prescribed anticoagulants as protection from venous thromboembolism after orthopedic surgery. The challenges faced by neurosurgeons may include how to effectively reverse these agents in a timely manner, deciding what method is most appropriate, and determining whether to observe the patient or emergently take the patient to the operating room. This chapter briefly reviews the anticoagulants and antiplatelet agents that are currently used, identifies the reversal agents of each of these drugs, and discusses their mechanism of action, dose, and indications (Tables 14.1 and 14.2). Unfractionated heparin is the most commonly used inpatient anticoagulant in patients with neurologic disorders. It is a polyglycosaminoglycan that binds to exosite 2 on thrombin (factor IIa [FIIa]) as well as coagulation factor Xa (FXa).3 The preference for binding to FIIa over FXa is largely due to its molecular weight, which ranges from 15 to 30 kd. The activity of heparin is measured using an activated partial thromboplastin time (aPTT). It has a relatively short half-life (t½) of 1 to 2 hours; therefore, after discontinuation of heparin, coagulation returns to baseline in approximately 3 to 4 hours. Heparin can be reversed using protamine, a positively charged molecule that binds to negatively charged heparin, preventing it from binding to thrombin. The effective dose of protamine is 1 mg per 100 IU of heparin. Protamine should be dosed based on the amount of active heparin in circulation, so if 1000 IU of heparin has been administered over 2 hours, patients should receive 20 mg of protamine in order to fully reverse the activity of heparin. Low molecular weight heparin (LMWH) is fractionated form of heparin with a molecular weight of 8 to 15 kd.3 The shorter length results in preference for FXa over FIIa. The half-life of LMWH is approximately 4 hours, so it usually takes several hours to return to a normal coagulation profile after drug administration. Protamine is also used to reverse the activity of LMWH, but it is only effective in reversing approximately 50% of the anti-Xa activity.4 Protamine is administered at a dose of 1 mg for every 1 mg dose of LMWH administered over the previous 6 to 8 hours.5 There are two classes of FXa inhibitors: pentasaccharides and an oral direct inhibitor of FXa. The two available pentasaccharides are fondaparinux and idraparinux, which has been discontinued. Fondaparinux has a shorter half-life of 17 hours6 compared with idraparinux, which is almost 80 hours.7 The only effective means of reversing the activity of these compounds is administering recombinant factor VIIa (rFVIIa), with an initial dose of 80 μg/kg.8,9 Rivaroxiban is an orally available direct FXa inhibitor with a half-life of 7 to 11 hours.10 It does not require monitoring, but it will increase the prothrombin time (PT). Reversal of this compound can be achieved using prothrombin complex concentrate, administering it intravenously and following either a PT or an anti-FXa assay.11 These compounds bind to the active site of thrombin and include both intravenous and oral forms and univalent and divalent classes. Argatroban is an intravenous, univalent formulation with a half-life of 50 minutes.12 Dabigatran is an orally available drug with a half-life of 12 to 17 hours.13 Bivalirudin, lepirudin, and desirudin are bivalent intravenous drugs that have half-lives of between 25 and 75 minutes.14 Unfortunately, this is the only class of anticoagulants that cannot be reversed by available agents, including rFVIIa15 and prothrombin complex concentrate (PCC).11 The bivalent, intravenous direct thrombin inhibitors should immediately be discontinued to return to baseline coagulation function, which can be verified by determining the thrombin time. Hemodialysis has been used to help with the reversal of dabigatran.16 The discovery of this class of anticoagulant dates back to the 1920s, when scientists observed that cattle that ingested a particular type of clover would die of a hemorrhagic illness.17 Subsequent work led to isolating a compound, called coumarin, that prevents blood clotting. Today, warfarin is the most commonly prescribed oral anticoagulant, although it is associated with an increased risk of intracerebral hemorrhage (ICH) and ICH-related mortality. It inhibits vitamin K–dependent clotting factors in the liver, including factors II, VII, IX, and X, as well as protein C and S. Warfarin belongs to a class of compounds called coumarins that contain 4-hydroxycoumarin, which inhibits vitamin K epoxide reductase, leading to inhibition of the above-mentioned clotting factors. Other drugs in this class include acenocoumarol, phenprocoumon, and anisindione. The half-lives of these compounds range from 18 hours to 10 days.18 Coumarin levels are followed by testing the prothrombin-time international normalized ratio (PT-INR or INR). The reversal strategy for coumarins depends on the urgency of reversal. Elective reversal entails using intravenous vitamin K at a dose of 1 to 2 mg administered by subcutaneous injection every 12 hours. There is no proven advantage to intravenous administration unless the patient is experiencing hemodynamic problems (shock). The reversal effect begins within 2 hours of the first injection, and reversal is usually achieved in 12 to 16 hours.19 Oral administration of vitamin K can take over 24 hours to reverse the activity of coumarin. For urgent reversal, fresh frozen plasma (FFP) or PCC is appropriate. The concentration of clotting factors in PCC is 60-fold higher than that in FFP,20 and PCC also has a more sustainable reversal phenotype. PCCs include inactivated plasmaderived concentrates of factor IX, with varying amounts of factors II, VII, and X, and protein C and S, and they work rapidly to reverse anticoagulation by replacing the vitamin K–dependent factors. The use of FFP depends on the time required to obtain the ABO blood type. PCCs are reconstituted quickly and can be given via an intravenous bolus over 2 to 5 minutes. A drawback of PCC is that it can lead to a hypercoagulable state, placing the patient at risk of arterial or venous thrombosis. Although vitamin K and FFP are routinely used to reverse anticoagulation, neither provides rapid INR reversal. Therefore, other options have been explored, including PCC, described above, and rFVIIa. If an emergency reversal in the operating room is needed, rFVIIa can be administered. It is a vitamin K–dependent glycoprotein that produces hemostasis by activating the extrinsic pathway. Recombinant FVIIa has a rapid onset of action and a short half-life of 2 to 3 hours.21 It is administered at an initial dose of 80 μg/kg, based on research evaluating doses to limit the size of the blood clot in patients with acute intracranial hemorrhage.22 It is still important to follow-up with administering FFP or PCC as well as vitamin K after the rFVIIa has been given. Although the INR reduces quickly, it will probably not be sustained, given the short duration of action, and rFVIIa does not replace other vitamin K–dependent clotting factors inhibited by warfarin. It is not as clear, however, how frequently to repeat the rFVIIa doses. A concern associated with rFVIIa is its potential to lead to acute thromboembolism. Mayer et al22 found that 80 µg/kg of rFVIIa was associated with a 5% absolute increase in the frequency of serious arterial thromboembolic events, despite the exclusion of patients thought to be at high risk. Aspirin is a salicylate that was synthesized in 1899 by Bayer originally to treat rheumatism.23 Its antiplatelet activity results from irreversibly binding to cyclooxygenase-1 (COX-1), thereby inhibiting thromboxane A2 (TXA2), which is an important catalyst in platelet activation. It works within minutes of ingestion, and although it has a relatively short half-life of 3 hours, its pharmacokinetic profile renders it effective for the life of the platelet (7–10 days). The standard of measurement in platelet inhibition is a platelet function analyzer, PFA-100. This is an ex-vivo whole blood assay that aspirates blood through an aperture on a collagen surface and measures the closing time. The normal closing time is between 60 and 120 seconds, depending on whether a collagen epinephrine cartridge or collagen adenosine phosphate (ADP) is used.24 When possible, it is best to avoid the use of aspirin for 7 to 10 days before elective surgery unless the clinical condition requires continued administration (e.g., carotid endarterectomy). To reverse the activity of aspirin, two strategies are employed. The first is administration of platelets with the goal of replacing aspirin-bound platelets with free circulating cells. Recently, a study found that administering platelets to patients with traumatic brain injury who are taking aspirin showed a dose-response relationship in reversing platelet inhibition.25 The second method is administration of deamino-D-arginine vasopressin (DDAVP), which enhances platelet adhesion by releasing FVIII and von Willebrand factor (vWF), which are essential factors in platelet adhesion.26 The dose of DDAVP is 0.3 μg/kg in saline administered over 30 minutes. Clopidogrel and prasugrel are thienopyridine molecules that inhibit platelet function by binding to the ADP receptor P2Y12. The PFA-100 can be used to evaluate platelet inhibition using collagen ADP cartridges.24 The strategy for reversal is similar to that for aspirin, using both platelet and DDAVP at a dose 0.3 μg/kg.27,28 Glycoprotein IIb/IIIa (GPIIb/IIIa) is an integrin found on the surface of platelets and, once activated, it converts from a quiescent to activated structure and binds fibrinogen, resulting in platelet aggregation.29,30 Abciximab, a chimeric human-murine monoclonal antibody was the first GPIIb/IIIa antagonist developed. Eptifibatide, a small peptide, interacts with and inhibit the function of the β3-subunit of GPIIb/IIIa. This subunit specifically recognizes an arginine-glycine-aspartic acid (AGD) residue on proteins such as vWF and fibrinogen and binds to it to form a platelet aggregate.31 Abciximab has a relatively short half-life but has high affinity for GPIIb/IIIa, resulting in platelet inhibition that can take 4 to 5 days to return to normal. Eptifibatide has a relatively low affinity for its target, such that the antiplatelet effect usually diminishes within hours after discontinuing the intravenous infusion, provided that the patient has normal renal function. Reversal strategies for GPIIb/IIIa inhibitors involve stopping administration of the drug and administration of platelet concentrate and fibrinogen by FFP or PPC.32 A PFA-100 can be used to follow platelet inhibition and reversal. Pathological thrombosis results in cardiovascular, cerebrovascular, and peripheral vascular disease, and represents the most common cause of morbidity and mortality in the Western world. Anticoagulant and antiplatelet therapy have improved the outcome in these patient populations.33 These drugs, however, do not come without significant risk, chief among them being hemorrhage.34 When patients present with hemorrhage or with the need for other emergent or urgent neurosurgical treatment, be it in the brain or spine, urgent anticoagulant or antiplatelet reversal is usually required. The reversal for both anticoagulant and antiplatelet drugs also carries risks, including venous thromboembolism, stroke, and myocardial infarction. This is further complicated in the setting of combined aspirin-Plavix therapy in patients who have intracranial or cardiac stents. Neurosurgeons must weigh the risk of reversal with the potential morbidity and mortality of not treating the patient with an intracerebral or intraspinal hemorrhage. KEY POINTS • Patient history, PT, PTT, and platelet count are clinically valuable when screening adult neurosurgery patients for the risk of postoperative bleeding. • Platelet count determination is clinically valuable for adult patients to rule out thrombocytopenia. • Prothrombin time (PT) assesses the extrinsic and common pathways of clotting and is typically expressed in the international normalized ratio (INR) format. • Activated partial thromboplastin time (aPTT) tests the integrity of the intrinsic and common pathways of coagulation. • Routine PT and PTT assessment are typically recommended to screen for problems of hemostasis in neurosurgery patients undergoing surgery, but the value of routine screening has not been validated in any randomized trials. Although recommended, they may at most provide baseline values. • A disseminated intravascular coagulation (DIC) panel is not typically used but may be required if there are indicators that suggest uncontrolled coagulation or bleeding. The DIC panel includes D-dimer (which increases), fibrinogen level (which decreases), and fibrinogen degradation products (FDPs, which increase). Platelet count also decreases. • Coagulation screening tests can be meaningfully interpreted only with the knowledge of their limitations and the relevant clinical situation.
Specific Anticoagulant and Antiplatelet Agent Reversal Strategies for Neurosurgical Patients
Anticoagulant Agents
Heparins
Factor Xa Inhibitors
Direct Thrombin Inhibitors
Vitamin K–Dependent Clotting Factor Inhibitors
Antiplatelet Agents
Nonsteroidal Anti-Inflammatory Drugs
Thienopyridines
Glycoprotein IIb/IIIa Inhibitors
Conclusion
Specific Anticoagulant and Antiplatelet Agent Reversal Strategies for Neurosurgical Patients
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree