Fig. 20.1
(a) Vascularization of the spinal cord. 1 Radiculomedullar artery (syn.: nervomedullar artery); 2 posterior radicular artery; 3 anterior radicular artery; 4 anterior spinal artery (ASA); 5 duplication of the anterior spinal artery, 6 sulcal artery (syn.: central artery, sulcocommissural artery); 7 posterolateral spinal artery (PLSA); 8 posterior spinal artery (PSA); 9 vasocorona; 10 transverse and longitudinal interconnections; 11 anterior nerve root; 12 dorsal nerve root with sensoric spinal ganglion. (b–f) Different infarct types and presumed vascular territories. (b) Anterior spinal artery (ASA) territory infarct (syn.: centromedullar infarct; ASA syndrome). (c) Posterior spinal artery (PSA)/posterolateral spinal artery territory infarct. (d) Artery of Adamkiewicz territory infarct. (e) Infarct in the sulcal artery territory (spinal sulcal artery syndrome). (f) Watershed infarcts between intrinsic and extrinsic system at the level of the anterior horns (“man-in-the-barrel” syndrome)
The radiculomedullary artery may divide into an anterior and a posterior radicular artery, but most often in man there is only one branch running with the dorsal or ventral nerve root [2, 4]. Three types of radicular arteries could be defined: (1) some radicular arteries (RA) end with the nerve root at the level of the dura mater without reaching or supplying the spinal cord, (2) other RA do not enter the surrounding arterial system of the spinal cord (vasocorona) and (3) another type of RA feeds the spinal vascular system. In total, there are 31 pairs of RA and the distribution of the 10–23 (mean: 12–16) posterior RA over the spine is homogeneous without lateral preference. The posterior RA supply the pial arterial plexus (vasocorona) of the lateral and dorsal spinal cord, the dorsal nerve roots and the sensoric spinal ganglia. As mentioned above, the number and locations of posterior RA are different to the anterior RA. Only in a few segments is the common extradural trunk, i.e. the radiculomedullary artery, branching into an anterior and posterior RA (Fig. 20.1a) [2, 4].
In contrast to the posterior RA, the number of the anterior RA is related to segments of the spinal cord [1–4]. The mean number in the cervical part is 0–6, at the thoracic part 1–4 and at the thoracolumbar level 1–2 [4].
In the lower thoracic and thoracolumbar region there is one dominant anterior RA, the arteria radicularis magna or artery of Adamkiewicz, with a diameter of 1.0–1.3 mm, in comparison with the other anterior RA with a mean diameter of 0.2 mm. About 75 % of arteries of Adamkiewicz originate from the aorta at levels Th 9–12, 10 % at levels L1–L2 and often the artery is left sided (80 %). The artery of Adamkiewicz has two branches, the smaller ascending and the larger descending branch, the latter supplying the lower thoracic and the lumbar part of the spinal cord including the conus medullaris. Therefore there is a greater flow of blood in the downward branch of the artery of Adamkiewicz [4].
20.1.1 Extrinsic Spinal Cord Arteries
20.1.1.1 Anterior Spinal Artery (ASA)
At the craniocervical level, the confluence of two intradural feeders arising from the distal VA, i.e. the V4-segment forms the ASA, which runs downwards in the anterior spinal fissure (see Fig. 20.2). However, the two vertebral branches may not fuse with the consequence of a double ASA. In this case, each of the two ASA supplies one inner half of the spinal cord via sulcal arteries (syn.: central artery or sulocommisural artery) (see Fig. 20.1a). The size of the ASA is variable because of the different number and calibres of the anterior RA, especially at the level of the artery of Adamkiewicz. Beside missed fusion of the cranial VA feeders, segmental duplication of the ASA for a short distance is frequent (50 %) (see Fig. 20.1a) [2, 4].
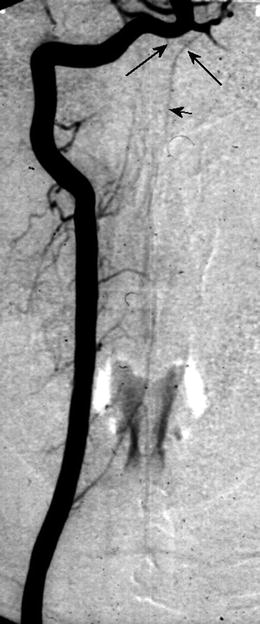

Fig. 20.2
Digital subtraction angiography (DSA, posterior anterior projection) of the right VA showing bilateral feeders from the V4 segment (arrow) fusing to the ASA (short arrow)
20.1.1.2 Posterior Spinal Artery (PSA)
The dual posterior spinal artery (PSA) also arises from the distal intradural V4 segments and may have additional feeders originating from the posterior inferior cerebellar artery (PICA) or from the rope ladder-like collateral vascular system of the medulla oblongata. With variable calibre there are, in addition, dual posterior lateral spinal arteries (PLSA) and these four arteries supply the dorsal and dorsolateral transverse section of the spinal cord (see Fig. 20.1a) [2, 4].
20.1.2 Extrinsic and Intrinsic System
The vasocorona is a circumference transverse and longitudinal collateral network on the surface around the spinal cord connecting the ASA, the PSA and the PLSA. Beside the ASA and PSA/PLSA territories, vascular supply of the spinal cord could be separated into extrinsic and intrinsic systems. The circumference of the spinal cord is supplied by numerous small penetrating arteries originating from the vasocorona, running directly perpendicular to the surface into the spinal cord representing the extrinsic or peripheral vascular system (see Fig. 20.1a) [1–4].
20.1.3 Sulcal Arteries (Syn.: Central or Sulcocommissural Arteries)
The sulcal arteries (SA) originate from the ASA and enter the spinal cord in the anterior fissure and represent the intrinsic central vascular system. Within the spinal cord they run alternating to the left or right side. However, when there is a duplication of the ASA, the SA supply only the side of the ASA from which they originate. There may be a common trunk of the SA with bifurcation in the sagittal plane in 7–9 % but never in the transverse sections. The number and size of SA depend upon the extent of the grey matter, especially at the level of the cervical and thoracolumbar intumescences of the spinal cord (Fig. 20.1a) [2].
Therefore, in the cervical section there are 5–8 SA and in the thoracolumbar region 5–12 SA per centimetre in the sagittal direction, whereas at the thoracic level with a smaller amount of grey matter the number of SA at 2–6 per centimetre is lower. In consequence, in the thoracic region the intramedullary course of the SA in sagittal plane is much more longitudinal, resulting in traversing branches up to 3 cm in length in comparison to the cervical and thoracolumbar section, whereas the SA has a more horizontal intramedullary course [2, 4].
A watershed region is also described at the level of the anterior and posterior horns, and there is no fixed border between the extrinsic or peripheral system and the intrinsic, i.e. central vascular system [2]. Consecutively there is an overlap and intermediate region nearby the anterolateral horns and the central part of the dorsal horns which is variably supplied by one system or the other. Regarding the perfusion of the spinal cord, the vasocorona with the penetrating perforators exhibit a centripetal bloodflow and the SA represent a centrifugal vascular system [4].
However, there is no fixed watershed region and the so-called “dead point” between neighbouring radicular arteries or the intrinsic and extrinsic system with blood flow in neither direction changes constantly over time [4, 5].
The capillary bed in the grey matter of the spinal cord is up to a five times densely because of the higher oxygen uptake. Especially the anterior horns with numerous motorneurons and the base of the posterior horns including the substantia gelantinosa need a thick capillary bed [4, 6]. The course of the capillaries in the white matter is be geared to the orientation of the fibre tracts. Whereas circumscribed loss of grey matter may be tolerated without neurological deficits, segmental loss of white matter may cause functional diachisis of fibre tracts, resulting in distinct clinical disorders.
However, in contrast to cerebral ischemia, spinal cord infarcts are rare because of the good collateral vascular supply.
20.2 Aetiology of Spinal Ischemia
Aetiology of spinal cord infarction is heterogeneous, including spontaneous or traumatic uni- or bilateral vertebral artery (VA) dissection [7–9], hypotension, e.g. caused by cardiac failure [7], fibrocartilaginous embolism [10–12], cocaine misuse [13, 14], arteriosclerosis of the VA and cardioembolic occlusion of the VA (see Table 20.1) [7, 9]. In the elderly, arteriosclerosis of the aorta and infrarenal aneurysm repair with potential occlusion of the artery of Adamkiewiecz are considerable risk factors for medullar ischemia. Further etiologies are vasculitis, e.g. panarteriitis nodosa or antiphospholipid antibody syndrome, neurosyphilis, spinal decompression sickness after diving, scoliosis operation with erection of the spine, diagnostic blockade of cervical nerve roots, spinal tumours and spinal arteriovenous malformation AVM (see Table 20.1). However, in about one third of spinal cord infarcts, aetiology remains unclear (idiopathic) [9].
Table 20.1
Aetiologies of spinal cord infarcts
Aortal surgery |
Systemic arteriosclerosis (risk factors, e.g. hypertension, diabetes) |
Dissection (aorta, VA dissection – uni/bilateral) |
Hypotension (cardiac failure) |
Cocaine |
Vasculitis |
Fibrocartilaginous embolism |
Embolic occlusion of the VA |
Diagnostic blockade of cervical nerve roots |
Spinal decompression sickness |
Scoliosis operation |
Compression of a lumbar artery |
Spinal AVM, dural fistula |
Spinal tumour |
Idiopathic |
20.3 Clinical Symptoms
20.3.1 Syndrome of the Anterior Spinal Artery (ASA)
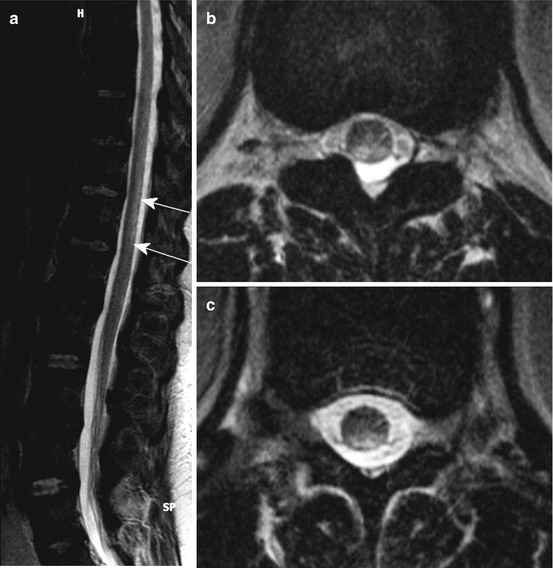
Ischemia in the ASA territory, including the SA with bilaterally and sometimes asymmetric lesions of the anterior horns, the lateral corticospinal tract, the thalamic pathways and the area around the central canal (commissural pathways), causes the “centromedullar syndrome” or syndrome of the ASA (see Fig. 20.1b and 20.3). This dramatic clinical feature is characterized by spastic para- or tetraparesis, dissociated sensation deficits with loss of pain and temperature sense below the lesion level of the spinal cord. In addition, there are bladder and bowel dysfunction, and when the vasoconstrictor tract in the lateral medullary part is affected, neurological syndrome including different temperature of the limbs and ipsilateral Horner’s syndrome in the case of cervical infarction. As a consequence of direct lower motor neuron damage, floppy paresis at the level of infarct occurs (Figs. 20.3) [3].
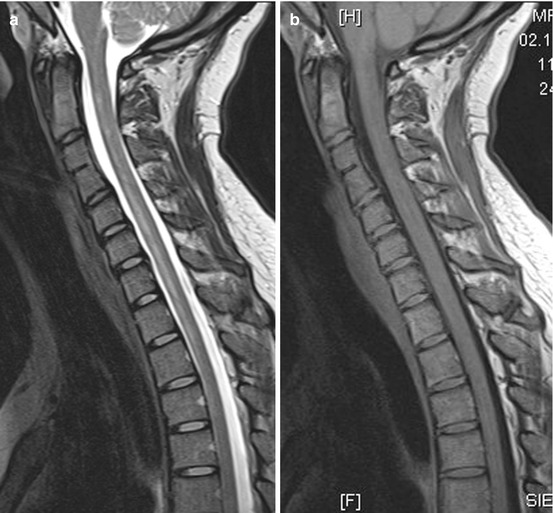
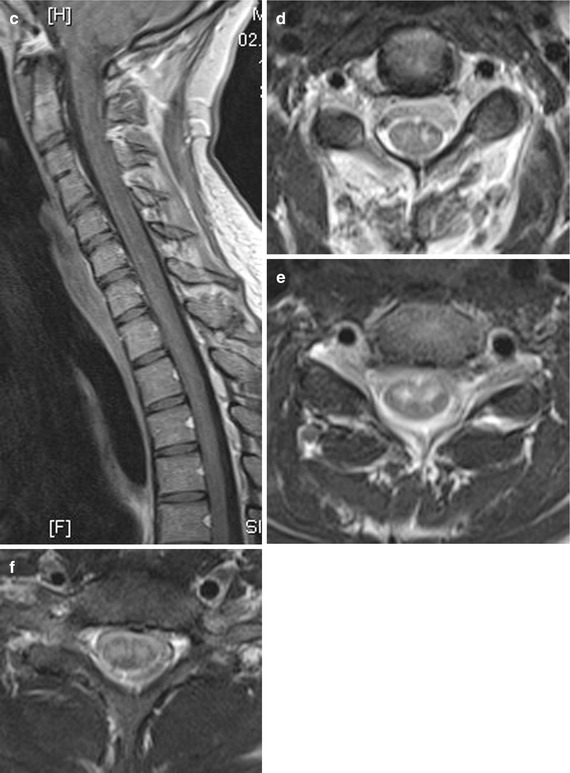


Fig. 20.3
(a–f) A 24-year-old woman suffering from ASA syndrome and rapid progressive tetraparesis within 3 h. Sagittal T2 WI (a) showing a “pencil-like” longitudinal hyperintense medullary lesion ventrally accentuated. Axial T2 WI (d–f) disclosing at the level C2/3 bilateral nearly symmetrical hyperintense signal changes probably in the anterior horns (d; “snake eyes”), which increase at level C4 (e) and lastly affect large parts of the transverse section sparing the PSA territories. T1 WI sag. before (b) and after contrast medium application (c) revealing slight inhomogeneous enhancement
However, in contrast to cerebral infarction with acute onset, the clinical deficits caused by spinal cord ischemia manifest over 30–45 min, accompanied by a radicular belt-like pain representing the start of this severe neurological disease.
20.3.2 Syndrome of the Posterior Spinal Artery (PSA)
Due to dual posterior (PSA) and posterolateral (PLSA) spinal arteries in combination with a pial collateral network, infarcts are more often located in the ASA territory than in the PSA/PLSA territories (see Fig. 20.4 and 20.1c) [15]. Disturbance of proprioception reflecting light touch and vibration sense due to damage of the dorsal columns causes atactic gait disturbances.