Chapter 6 Spinal Cord Stimulation
Implantation Techniques
 No studies exist to validate a particular trialing technique over another, although advantages and disadvantages of each approach exist.
No studies exist to validate a particular trialing technique over another, although advantages and disadvantages of each approach exist. Either percutaneous cylindrical leads or surgically placed plate leads are used for permanent implantation.
Either percutaneous cylindrical leads or surgically placed plate leads are used for permanent implantation. Plate leads are less likely to migrate and are less susceptible to position but require a more invasive surgery for implantation.
Plate leads are less likely to migrate and are less susceptible to position but require a more invasive surgery for implantation. The generator pocket should be deep enough to avoid erosion but superficial enough for interrogation and recharging.
The generator pocket should be deep enough to avoid erosion but superficial enough for interrogation and recharging. Careful planning is essential to successful outcomes when utilizing SCS systems. Planning includes appropriate patient selection; trialing technique; lead type and location; and generator type and location.
Careful planning is essential to successful outcomes when utilizing SCS systems. Planning includes appropriate patient selection; trialing technique; lead type and location; and generator type and location. Trialing and implant techniques vary among practitioners. Each technique carries unique advantages and disadvantages. Techniques should be carefully selected by practitioners based on their experience and individual patient characteristics.
Trialing and implant techniques vary among practitioners. Each technique carries unique advantages and disadvantages. Techniques should be carefully selected by practitioners based on their experience and individual patient characteristics. Optimal lead placement seeks to achieve non-painful paresthesias in the areas of pain. This typically occurs in the posterior epidural space, near midline, at a spinal level common for a particular pain distribution.
Optimal lead placement seeks to achieve non-painful paresthesias in the areas of pain. This typically occurs in the posterior epidural space, near midline, at a spinal level common for a particular pain distribution. Lead anchoring must be carried out meticulously to minimize migration. This involves redundant suturing techniques and utilization of optimal fascia.
Lead anchoring must be carried out meticulously to minimize migration. This involves redundant suturing techniques and utilization of optimal fascia. Fluoroscopic imaging in both AP and lateral views should be utilized frequently to ensure patient safety and device stability during placement.
Fluoroscopic imaging in both AP and lateral views should be utilized frequently to ensure patient safety and device stability during placement. Generator location selection should be individualized based on each patient’s anatomy and the stimulator position.
Generator location selection should be individualized based on each patient’s anatomy and the stimulator position. Practitioners who choose to implant SCS systems should be prepared to manage the potential complications; this includes SCS repositioning, revision, and explantation.
Practitioners who choose to implant SCS systems should be prepared to manage the potential complications; this includes SCS repositioning, revision, and explantation. Lead damage can occur both during placement and post-placement. To minimize future damage, one must appreciate the lead location relative to the supraspinous and interspinous ligaments.
Lead damage can occur both during placement and post-placement. To minimize future damage, one must appreciate the lead location relative to the supraspinous and interspinous ligaments. Physiological trespass in the neuraxial space can have devastating consequences. Meticulous care should be taken at all times regarding surgical and aseptic technique.
Physiological trespass in the neuraxial space can have devastating consequences. Meticulous care should be taken at all times regarding surgical and aseptic technique. Lead migration and signal interruption can manifest following poor anchoring techniques, insufficient lead length, and excessive use of extensions.
Lead migration and signal interruption can manifest following poor anchoring techniques, insufficient lead length, and excessive use of extensions.Patient Selection
Patient selection for SCS is reviewed in detail elsewhere in this book. The ideal candidates for percutaneous leads are younger patients who do not have significant degenerative spine disease or pronounced scoliosis and/or kyphosis. The patients depicted in Figs. 6-1 and 6-2 have significant scoliosis and were considered potentially difficult percutaneous placements. Approaching from the convex side of the scoliotic curve, the trials proceeded uneventfully as did the subsequent permanent percutaneously placed epidural leads.
Migration of percutaneously placed spinal cord stimulator leads has been reported in many studies.1–3 The reported incidence ranges from 5% to 23% in different series.1–4 Proper patient selection should help to minimize the likelihood of subsequent migration. As with morbidly obese patients, very thin patients may prove more technically challenging for the percutaneous implanter. This may include finding appropriate space for the generator and anchors and fixation of the leads.
Trialing
How much pain relief is necessary before considering a trial successful for subsequent permanent implant? The literature often reports 50% pain relief as an outcome for judging a successful trial.5–6 No good studies have looked at whether a criterion of 50% pain relief during a trial period predicts long-term success with SCS. It is quite possible that some patients with less than 50% relief may find acceptable relief long term and/or significant improvement in activities of daily living and increased functional abilities. It is known that some patients who report 50% or greater pain relief during a trial do not sustain relief long term and eventually become therapy failures.
Risk of infection during spinal cord stimulator trial has been infrequent.7–8 Meticulous sterile technique should be followed during the trial placement. Literature from other implantable trial catheters suggest that the risk of infection increases with the duration of the trial.9 A recent study with intrathecal catheters (in which the risk of serious neuraxial infection would be expected to be greater than epidurally placed spinal cord stimulator leads) reported no infections until week 3 and thereafter an incidence of 16% for catheters placed longer than 2 weeks.9
Positioning the Spinal Cord Stimulator Lead
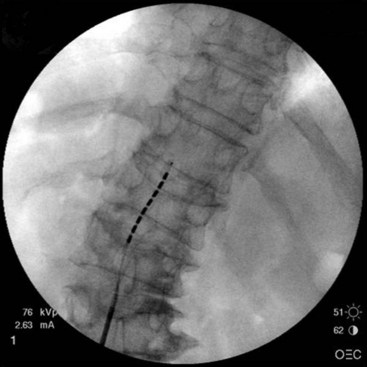
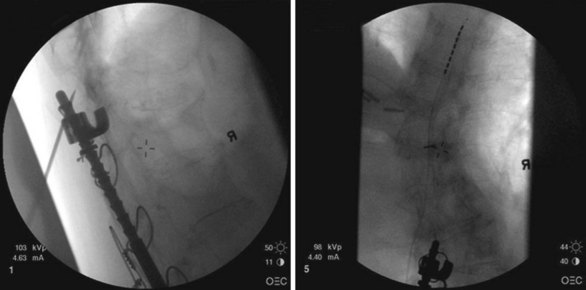
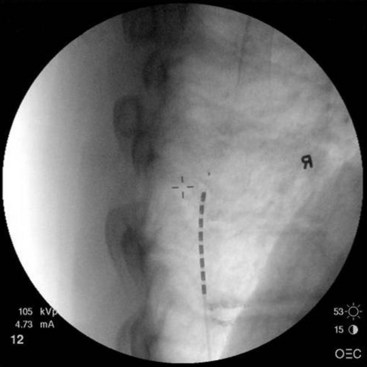
After sterile surgical preparation (e.g., chlorhexidine), many implanters use an Ioban drape over the surgical site. Standard sterile surgical techniques are used. Needle entry for percutaneous placement depends in part on anticipated final placement of the lead(s). Common needle entry for the lower extremity and/or axial low back pain is the midlumbar region. Skin entry commonly is marked at L2-3, L3-4, or L4-5. Entry into the epidural space should be as flat as possible, dependent in part on the body habitus of the patient. Entry into the epidural space is either one or often two levels above skin insertion. A paramedian approach should be used to avoid both the forces of the supraspinous and interspinous ligaments and the tendency of the spinous process to fracture a lead placed through a midline approach. The percutaneous implanter should not hesitate to use a longer-than-standard epidural needle to ensure that the angle of approach to the epidural space is shallow (less than 45 degrees whenever possible). A lateral view should be taken to ensure that the lead has not migrated anteriorly in the epidural space or into the dura (Fig. 6-3).
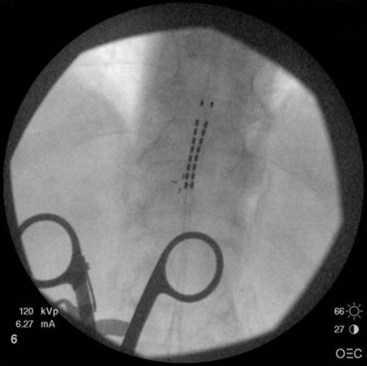
Common lead placement for lower-extremity paresthesias vary from T9 to T12 (Figs. 6-4 and 6-5). Lead placement below T12 will not consistently stimulate posterior columns since the spinal cord often terminates at L1 or L2. Stimulation for axial back paresthesias commonly requires placement of the leads at T7 and/or T8. As stated previously, final lead placement should always be individualized to the patient response during intraoperative mapping.
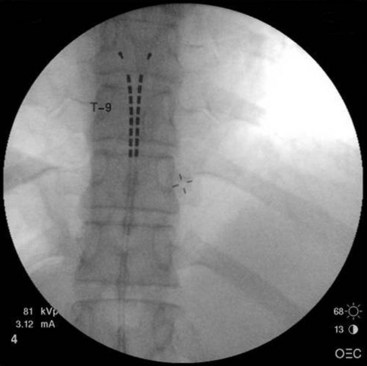
Fig. 6-4 Lead placement for typical spinal cord stimulation of lower extremities and axial back pain.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree