Chapter 10 Spinal Cord Stimulation for Peripheral Vascular Disease
 SCS improves pain, microcirculation, limb survival, and clinical stage compared to conservative treatment.
SCS improves pain, microcirculation, limb survival, and clinical stage compared to conservative treatment. When selecting patients, the improvement in the transcutaneous partial pressure of oxygen (TcpO2) and pain relief during trial stimulation is a predictor of outcome.
When selecting patients, the improvement in the transcutaneous partial pressure of oxygen (TcpO2) and pain relief during trial stimulation is a predictor of outcome. Increase of TcpO2 of 10 mm Hg (minimum), preferably >15 mmHg, is a prognostic indicator for both limb salvage and pain relief; however, there is no linear correlation.
Increase of TcpO2 of 10 mm Hg (minimum), preferably >15 mmHg, is a prognostic indicator for both limb salvage and pain relief; however, there is no linear correlation. If the regional perfusion index (RPI) improves 0.2 or more from the baseline and is sustained, the limb salvage rate could reach up to 90%.
If the regional perfusion index (RPI) improves 0.2 or more from the baseline and is sustained, the limb salvage rate could reach up to 90%. If SCS is implanted in cases where trial stimulation produces less than 80% coverage of the area of pain, results will be less than satisfactory.
If SCS is implanted in cases where trial stimulation produces less than 80% coverage of the area of pain, results will be less than satisfactory. If TcpO2 does not rise over 100 mm Hg during trial stimulation, installation of an SCS in such cases will not produce improvement in rest pain, claudication, or limb salvage.
If TcpO2 does not rise over 100 mm Hg during trial stimulation, installation of an SCS in such cases will not produce improvement in rest pain, claudication, or limb salvage.Establishing Diagnosis
Peripheral vascular disease (PVD) results from progressive atherosclerosis of the arteries of the lower extremities. The presence of PVD signals an increased likelihood of disease in other regions of the body, resulting in cardiovascular and cerebrovascular morbidity and mortality. PVD affects 10% to15% of the U.S. population; the prevalence increases with advancing age.1 Risk factors for PVD include smoking, diabetes, hypertension, dyslipidemia, age greater than 40 years, being of African origin, and previous cardiac and cerebrovascular disease.
 The individual with neurogenic claudication who walks for a long time is able to walk shorter and shorter distances at the expense of longer and longer periods of rest. This is in contrast to the patient with vascular claudication, who can walk the same distance with equal rest periods in between.
The individual with neurogenic claudication who walks for a long time is able to walk shorter and shorter distances at the expense of longer and longer periods of rest. This is in contrast to the patient with vascular claudication, who can walk the same distance with equal rest periods in between.The severity of the symptoms in vascular claudication is governed by the amount of stenosis, presence of collateral circulation, and vigor of exercise. The classification systems used to stage severity of disease are the Fontaine and the Rutherford systems, with the Fontaine system used more widely (Table 10-1). Stenoses in the arterial tree secondary to atherothrombosis are responsible for the underlying pathophysiology of this disease. As the disease advances, the resistance in the vascular system increases, and the system is unable to provide oxygenation to the muscles, especially during exercise. This results in claudication, ischemia, ulceration, and eventual loss of limb if left untreated.2 Skeletal muscle ischemia affects muscle metabolism with the accumulation of lactate and intermediates of oxidative metabolism (acylcarnitines). This in turn causes muscle deterioration, denervation, and atrophy.3 Chronic ischemia leads to ulceration and gangrene. Pain resulting from ulcers and gangrene is caused by ischemic neuropathy and necrosis of the sensory nerves at the site of the lesion. Severe necrosis of the sensory nerves can paradoxically make gangrenous lesions insensate and anesthetic.
Table 10-1 Fontaine Classification of PAD
| Stage | Clinical |
|---|---|
| I | Asymptomatic |
| IIa | Mild claudication |
| IIb | Moderate-severe claudication |
| III | Ischemic rest pain |
| IV | Ulceration or gangrene |
Investigations
In the initial stages of PVD clinical history and physical examination are generally unreliable, with the diagnosis being missed more than 90% of the time based on these two factors alone. To make an early diagnosis of PVD, measurement of the ankle-brachial index (ABI) is helpful. The ABI is a ratio of the systolic blood pressure in the ipsilateral dorsalis pedis and posterior tibial arteries to that in the brachial artery (higher of bilateral brachial pressures), measured with a handheld continuous wave Doppler in the supine position. Normal ABI ranges between 1.0 and 1.3. An ABI <0.9 is 95% sensitive and 100% specific for PVD. Angiograms completed in these patients show more than 50% stenosis in one or more major blood vessels.4 The lower the ABI score, the more severe the PVD, with ABI <0.4 representing advanced ischemia. Segmental limb pressure, segmental volume plethysmography, duplex ultrasonography, computed tomography (CT) angiography, and magnetic resonance (MR) angiography are additional modalities for evaluation of the level and extent of disease (Fig. 10-1).
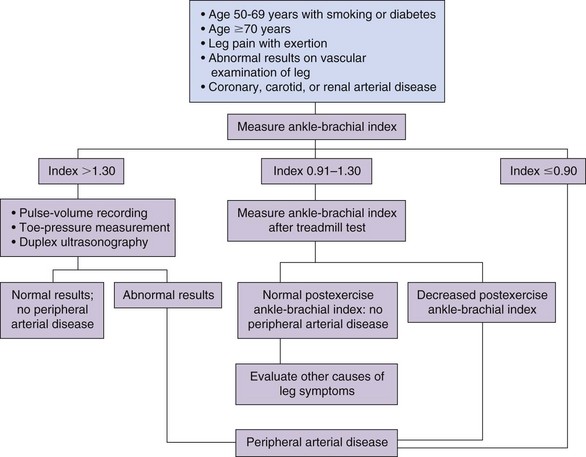
Fig. 10-1 Evaluation of patients with suspected peripheral vascular disease.
Adapted from Hiatt WR et al.: Medical treatment of peripheral arterial disease and claudication, N Engl J Med 344:1608, 2001.
The goals of treatment for patients with PVD are to relieve claudication, improve walking capacity, and improve quality of life. Initial management of PVD includes modification of risk factors such as smoking cessation, glycemic control, blood pressure normalization, and dyslipidemia management. Exercise rehabilitation has also been shown to be beneficial in reducing the symptoms of intermittent claudication by improving collateral circulation and thus improving functional status. Medical treatment of PVD includes antiplatelet and vasodilatory drugs. Revascularization is indicated when ischemic pain is severe, indicated by disabling claudication that prevents the patient from performing daily activities of living, rest pain, ischemic ulcers, or gangrene. Amputation is indicated in approximately 5% of patients with nonreconstructible critical limb ischemia and extensive tissue necrosis or life-threatening infection.5
Spinal cord stimulation (SCS) was first shown to be effective in relieving claudication symptoms and increasing blood flow to lower extremities by Cook and associates6 in 1976, who were using SCS to treat limb pain in patients with multiple sclerosis. During the late 1980s to early 1990s, the use of SCS as an alternative treatment measure for PVD rapidly advanced, especially in Europe. Currently SCS is the most promising neuromodulatory treatment for ischemic pain, and the overall beneficial effects last for at least 1 year in 80% of patients and for up to 5 years in 60% of patients.7 The beneficial effects of SCS in the treatment of ischemic pain include pain relief, ulcer healing, decreased oxygen requirement, and increased claudication distance.
Basic Science
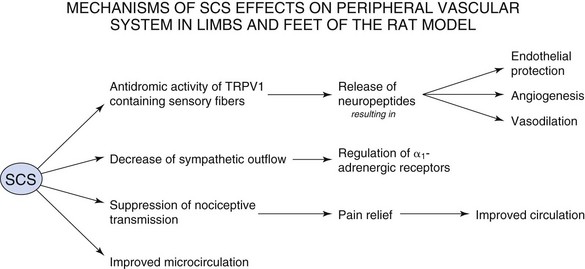
There are no established animal models with PVD that give rise to ischemic pain. Therefore normal Sprague-Dawley rats are used for research into the mechanisms of action of SCS for PVD. These rats are used to study acute changes in peripheral blood flow during SCS. SCS intensity in the rat is determined by the motor threshold (MT), which is the stimulation required for muscle contraction to be observed. Experimental SCS is performed at 30%, 60%, 90%, and 300% of MT. Stimulation at 30% MT is the minimum stimulus that produces vasodilation, with 60% MT being the level that approximates the stimulation parameters in clinical applications in humans. SCS at the upper lumbar spinal segments such as L2-L3 produces the largest increase in cutaneous blood flow in the lower limbs in the rat.8 There are several proposed theories as to the mechanism of action of SCS in the treatment of PVD:
 With regard to pain control, the action of SCS falls back on the Wall-Melzack gate control theory of pain, which proposes that stimulation of large diameter afferent fibers such as those in the dorsal columns of the spinal cord would close notional gates in lamina V of the dorsal horn. This would prevent the ascent of impulses that mediate pain and that originate in small-diameter afferents from ascending to higher levels.9 Relief from pain decreases the vasoconstriction that occurs as a reflex response.
With regard to pain control, the action of SCS falls back on the Wall-Melzack gate control theory of pain, which proposes that stimulation of large diameter afferent fibers such as those in the dorsal columns of the spinal cord would close notional gates in lamina V of the dorsal horn. This would prevent the ascent of impulses that mediate pain and that originate in small-diameter afferents from ascending to higher levels.9 Relief from pain decreases the vasoconstriction that occurs as a reflex response. SCS-induced vasodilation occurs via suppression of sympathetic activity. The sympathetic nervous system causes vasoconstriction via stimulation of α1– and α2-adrenoreceptors. Linderoth, Herregodts, and Meyerson10 observed that cutaneous vasodilation after SCS in the rat hind paw was eliminated by complete surgical sympathectomy. Administration of ganglionic blocker, hexamethonium, or neuronal nicotinic ganglionic blocker, chlorisondamine, had the same effect. High-dose adrenergic receptor blockers phentolamine and prazosin also suppressed SCS-induced vasodilation. Inhibition of vasodilation was not observed after administration of muscarinic receptor antagonists. However, there are conflicting data with the sympathetic mechanism because some patients demonstrate vasodilation with SCS even after chemical or surgical sympathectomy11 and some incompletely sympathectomized rats in Linderoth, Herregodts, and Meyerson’s study still retained the effects of SCS.
SCS-induced vasodilation occurs via suppression of sympathetic activity. The sympathetic nervous system causes vasoconstriction via stimulation of α1– and α2-adrenoreceptors. Linderoth, Herregodts, and Meyerson10 observed that cutaneous vasodilation after SCS in the rat hind paw was eliminated by complete surgical sympathectomy. Administration of ganglionic blocker, hexamethonium, or neuronal nicotinic ganglionic blocker, chlorisondamine, had the same effect. High-dose adrenergic receptor blockers phentolamine and prazosin also suppressed SCS-induced vasodilation. Inhibition of vasodilation was not observed after administration of muscarinic receptor antagonists. However, there are conflicting data with the sympathetic mechanism because some patients demonstrate vasodilation with SCS even after chemical or surgical sympathectomy11 and some incompletely sympathectomized rats in Linderoth, Herregodts, and Meyerson’s study still retained the effects of SCS. The antidromic mechanism was first proposed by Bayliss in 1901,12 who noted that dorsal root stimulation at high intensity induced peripheral vasodilation mediated by thin fibers. SCS antidromically activates afferent fibers in dorsal roots, causing the peripheral release of calcitonin gene-related peptide (CGRP), a powerful microvascular vasodilator. Since this time there has been intensive research into the types of fibers that mediate vasodilation and the vasodilators that are released during SCS. CGRP is found in small, myelinated Aδ fibers and unmyelinated C fibers in dorsal root ganglia. By stimulating at various thresholds and blocking C-fiber conduction with the application of capsaicin, Tanaka and colleagues13 determined that SCS-induced vasodilation at ≤60% MT is mediated by antidromic activation of myelinated fibers, whereas vasodilation at ≥90% is mediated by both myelinated and unmyelinated C-fibers.13 Wu and associates14 further characterized that SCS-induced vasodilation is predominantly mediated by those sensory fibers that contain transient receptor potential vanilloid-1 (TRPV1).14
The antidromic mechanism was first proposed by Bayliss in 1901,12 who noted that dorsal root stimulation at high intensity induced peripheral vasodilation mediated by thin fibers. SCS antidromically activates afferent fibers in dorsal roots, causing the peripheral release of calcitonin gene-related peptide (CGRP), a powerful microvascular vasodilator. Since this time there has been intensive research into the types of fibers that mediate vasodilation and the vasodilators that are released during SCS. CGRP is found in small, myelinated Aδ fibers and unmyelinated C fibers in dorsal root ganglia. By stimulating at various thresholds and blocking C-fiber conduction with the application of capsaicin, Tanaka and colleagues13 determined that SCS-induced vasodilation at ≤60% MT is mediated by antidromic activation of myelinated fibers, whereas vasodilation at ≥90% is mediated by both myelinated and unmyelinated C-fibers.13 Wu and associates14 further characterized that SCS-induced vasodilation is predominantly mediated by those sensory fibers that contain transient receptor potential vanilloid-1 (TRPV1).14Several vasodilators, including CGRP, are contained within the terminals of TRPV1 sensory nerve endings. Antidromic activation and depolarization of these nerve endings cause release of vasodilators into muscle tissue. CGRP is a potent vasodilator that is tenfold more powerful than prostaglandins and 100 to 1000 times more effective than other typical vasodilators such as acetylcholine, adenosine, and substance P.15 CGRP binds to CGRP-1 receptor of smooth muscle cells and causes direct relaxation or can bind to CGRP-1 receptor of endothelial cells, which causes release of nitric oxide, which leads to vasodilation. Adrenomedullin is a peptide that co-localizes with CGRP in perivascular nerves and dorsal root ganglia and is involved with angiogenesis and endothelial protection.8
Despite the intense research into the mechanisms of action of SCS for the treatment of PVD, the theories are incompletely understood, and there is much more to explore. It is very likely that reduction in pain transmission, the sympathetic theory, and the antidromic theory act in concert to provide pain relief and vasodilation in patients suffering from PVD. The relative weight of one mechanism over another may depend on the patient’s personal set of risk factors, sympathetic activity, and stimulation parameters. The mechanisms of SCS on the peripheral vascular system are summarized in Fig. 10-2.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree