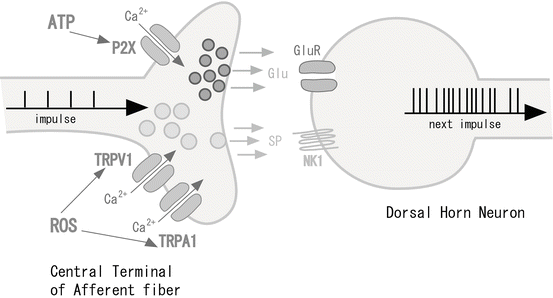
Fig. 31.1
Excitatory synaptic transmission in the dorsal horn. When an impulse is transmitted to central terminals of afferent fibers, excitatory neurotransmitters are released. Glutamate and substance P act at each receptor on the dorsal horn neuron and induce an impulse
31.2.2 Inhibitory Synaptic Transmission in the Spinal Cord
Although dorsal horn neuron action potential discharge is driven by glutamatergic EPSPs, the activity of these neurons is powerfully suppressed by inhibitory inputs in both the pre- and postsynapse. The inhibitory effect is mainly produced by γ-aminobutyric acid (GABA) and/or glycine postsynaptically, which are released from the terminals of inhibitory interneurons (Fig. 31.2). These interneurons induce fast inhibitory postsynaptic potentials (IPSPs) on dorsal horn neurons to mediate the activation of GABAA and glycine receptors. GABAA and glycine receptors are ligand-gated Cl− channels. Channel opening inhibits action potentials by hyperpolarizing the cell membrane. Activation of G-protein-coupled receptors produces postsynaptic inhibition of excitatory synaptic transmission to hyperpolarize cell membranes by activating K+ channels. This type of channel includes receptors such as the GABAB receptor, adenosine receptor, and opioid μ-receptor. Moreover, some endogenous substances suppress the release of glutamate from primary afferent terminals, therefore inhibiting discharge of dorsal horn neurons presynaptically. This presynaptic inhibition involves many of the same chemical mediators that cause postsynaptic inhibition, with receptors localized on the presynaptic terminals of primary afferents. There are not only networks within the spinal cord but also inhibitory projections to the dorsal horn from the brainstem, which is known as the descending inhibitory system. Serotonin [10] from the raphe nucleus, noradrenaline [10, 11] from the locus ceruleus, and dopamine [12, 13] from the hypothalamus A11 act on 5-HT1A receptors, α2 receptors, and D2-like receptors in dorsal horn neurons pre- and postsynaptically, respectively.
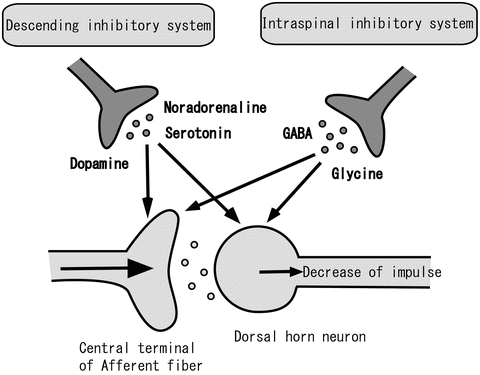

Fig. 31.2
The endogenous inhibitory system. The endogenous inhibitory system consists of the descending inhibitory system and the intraspinal inhibitory system
31.3 Spinal Synaptic Plasticity Induces Pathological Chronic Pain
There are two types of plasticity in the nervous system: one is central nervous sensitization, and the other is peripheral sensitization. In particular, central sensitization in the spinal cord has much to do with chronic pain. Although spinal synaptic plasticity induces pathological chronic pain, there are many causes and various forms of spinal synaptic plasticity. In recent years, the influential mechanisms involved in spinal synaptic plasticity have become clear. We describe some convincing mechanisms in the following explanation.
31.3.1 Change in Efficiency of Excitatory Synaptic Transmission
The change in efficiency of excitatory synaptic transmission by nervous activation plays an important role in the enhancement of pain, as well as memory and learning in the hippocampus. With respect to electrophysiological phenomena, the enhancement of efficiency in excitatory synaptic transmission occurs by windup and long-term potentiation (LTP). Windup is when noxious peripheral stimulation that is more intense or sustained induces primary afferent nociceptors to discharge at higher frequencies (Fig. 31.3①). LTP refers to high-frequency primary afferent stimulation that induces the potentiation of glutamate receptor-mediated responses at synapses onto dorsal horn neurons (Fig. 31.3②). Windup and LTP are considered to be involved in the release of peptide neuromodulators such as substance P, together with glutamate, from central terminal C-fibers. These peptides act on their G-protein-coupled receptors to produce depolarizing synaptic potentials lasting up to tens of seconds. Moreover, both phenomena are also considered to be related to the activation of NMDA receptors by phosphorylation via kinase, and the subsequent current flow through NMDA receptors leads to a rise in intracellular Ca2+ levels.
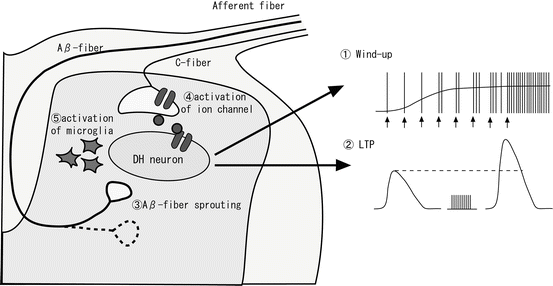

Fig. 31.3
Spinal synaptic plasticity. Spinal synaptic plasticity is induced by various causes. For example, ① windup, ② LTP, ③ Aβ-fiber sprouting into laminaII, ④ activation of ion channels on the central terminal of afferent fibers, and ⑤ activation of microglia
31.3.2 Alterations to the Synaptic Network in the Dorsal Horn
The synaptic network changes dynamically according to neural activity, for example, growth or regression of axons, the appearance of new synapses, or the disappearance of existing synapses. Alterations to the synaptic network (synaptic morphology change) in the dorsal horn have been suggested to lead to pain in chronic inflammation [14] or peripheral nerve injury. Under normal conditions, noxious information is transmitted through Aδ- and C-fibers to the superficial dorsal horn, especially substantia gelatinosa (SG) neurons (lamina II of Rexed), while innocuous mechanical information is transmitted through Aβ-fibers to the deep dorsal horn (lamina III–IV). In past studies, it was shown that C-fibers were missing in the sciatic nerve amputation pain model, and approximately 10 % of Aβ-fibers started to extend axons (sprouting), with input into lamina II [15, 16] (Fig. 31.3③). Moreover, Aβ-fiber sprouting was observed in the inflammation pain model [17]. Aβ-fiber sprouting in these pain models plays an important role in brain-derived neurotrophic factor (BDNF), which is released from C-fibers into the spinal cord. The mechanism of allodynia has been suggested to occur when innocuous mechanical information is converted to noxious information. This process occurs when Aβ-fibers connect to SG neurons, which project noxious information to the more central nervous system during pathological conditions such as chronic inflammation and neuropathic pain.
31.3.3 Neurotrophic Factors
Neurotrophic factors are supplied to nerve cells by many kinds of cells (e.g., glia cells, dorsal root ganglion (DRG) neurons, Schwann cells, keratinocytes, and fibroblasts) and have various influences on the transmission of noxious information. During inflammation, the production of nerve growth factor (NGF) increases and causes hyperalgesia. NGF interacts with the TrkA receptor at the peripheral terminals of nociceptive fibers and is carried to DRG neurons by a retrograde axial. The NGF-TrkA complex controls gene expression in DRG neurons and promotes the production of neuropeptides, excitatory ion channels, and BDNF. BDNF is transferred to the central terminal of nociceptive fibers, which are projected to the spinal cord, where it acts upon its receptor, TrkB, on dorsal horn neurons. The subsequent activation of TrkB receptors on dorsal horn neurons modulates pain information [18]. Furthermore, BDNF also contributes to the change in the synaptic network in the dorsal horn, as mentioned above, during inflammation and neuropathic pain.
31.3.4 Activation of P2X Receptors
Much attention has been given to ion channels in the central terminals of Aδ- and C-fibers, which enhance nociceptive pain information by increasing glutamate release when these channels are activated by endogenous mediators (Fig. 31.4). For example, these ion channels include the ATP-dependent P2X receptor, the transient receptor potential (TRP) receptor family, voltage-dependent Na+ channels, and acid-sensing ion channel 3 (ASIC3). In particular, we focused our attention on the P2X receptor in the dorsal horn. The P2X receptor family consists of at least seven P2X subunits (P2X1–P2X7). Each functional P2X receptor is composed of three or more P2X subunits, forming a pore structure that is permeable to cations including Ca2+. Therefore, the P2X receptor family is a ligand-gated ion channel family. We examined the role of P2X receptors in modulating excitatory synaptic transmission by using patch-clamp recordings from dorsal horn neurons of the rat spinal cord [6, 7, 19, 20]. Distinct subtypes of P2X receptors were located at central terminals of primary afferents that innervate onto lamina II and lamina V neurons. On activation, these P2X receptors enhanced the release of glutamate in various ways. In lamina II neurons, the modulation of glutamate release by presynaptic P2X receptors was mainly transient. In contrast, the P2X receptor-mediated modulation of glutamate release was relatively long lasting in lamina V neurons [20]. Pharmacological studies suggested that lamina II neurons were involved in homomeric P2X3 receptors, while lamina V neurons were involved in non-P2X3 receptors [20]. Differences among P2X-expressing afferent fibers innervating lamina II and lamina V neurons were also seen in terms of capsaicin sensitivity [19]. P2X-expressing afferent central terminals in lamina II were derived from capsaicin-sensitive primary afferents, while those in lamina V were from capsaicin-insensitive primary afferents [19]. Because P2X3-expressing afferent central terminals directly make synapses with lamina II neurons, the inputs from these afferent terminals could forward excitatory synaptic activities. On the contrary, the activities conveyed to lamina V neurons from P2X3-expressing primary afferents were polysynaptic; these inputs together with monosynaptic inputs from P2X-expressing and capsaicin-insensitive afferents were shown to converge on lamina V neurons. These results indicate that distinct subtypes of P2X receptors are expressed in central terminals of primary afferents innervating onto superficial and deep dorsal horn neurons and modulate glutamate release in a different manner. Furthermore, P2X receptors were localized at lamina V neurons to mediate postsynaptic sensory transmissions [21]. Using the whole-cell patch-clamp technique, we investigated whether the activation of postsynaptic P2X receptors can modulate synaptic transmission in lamina V neurons. The ATP analogue generated an inward current in lamina V neurons. These pre- and postsynaptic actions may serve to understand P2X receptor-mediated modulation of various sensations.