St. John’s Wort: A Critical Evaluation of the Evidence for Antidepressant Effects
Andrew A. Nierenberg
Hannah G. Lund
David Mischoulon
Saint John’s wort (SJW), Hypericum perforatum, has a long history of medicinal uses, including as an herbal preparation to exorcise evil spirits, as an antiviral agent, and more recently as a natural antidepressant. While many studies (mostly conducted in Europe) have supported the antidepressant effects of SJW, several key studies in the United States have, thus far, been negative (1). Since 2005, studies of hyperforin-enriched preparations have found that SJW is better than placebo and equal to standard antidepressants. Overall, evidence from clinical trials provides inconsistent data that SJW has efficacy for major depressive disorder (1). This chapter is a critical review of the preclinical and clinical evidence for SJW’s antidepressant efficacy, which focuses on the details of several key studies.
CANDIDATE MECHANISMS OF ACTION: HYPERICIN AND HYPERFORIN
SJW is a phytochemical nutraceutical antidepressant that could be viewed skeptically by clinicians accustomed to prescribing FDA-approved medications, particularly since no specific mechanism of action has yet been fully characterized for it. Nonetheless, the components of SJW do have physiological effects that are consistent with established antidepressants. SJW extracts contain various presumed active components such as polycyclic phenols, hypericin and pseudohypericin, and hyperforin. Other compounds that have been isolated from SJW include flavinoids (hyperoside, quercitin, isoquercitrin, rutin), kaempferol, luteolin, and biapigenin (2, 3, 4).
Hypericin
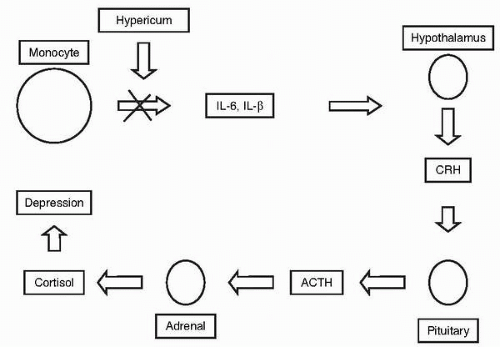
Hypericin, which is considered a principal active component of SJW, may function in part by decreasing serotonin receptor density (5). But because hypericin cannot cross the blood-brain barrier, one of its proposed mechanisms of action is through modification of inflammatory cytokines, consistent with findings that certain individuals with major depressive disorder have increased levels of inflammatory markers (6). SJW inhibits production of interleukin-6 and -1β by monocytes, which results in a decrease in corticotrophin-releasing hormone that in turn dampens cortisol production via the hypothalamus-pituitary-adrenal axis (7), thus countering the hypercortisolemia associated with stress and depressive states (Fig. 3.1). Hypericin may also inhibit reuptake of serotonin, norepinephrine, and dopamine (5), and may result in down-regulation of beta adrenoreceptors and increased 5-hydroxytryptamine-2 (5HT2) and 5HT1a receptor density (8); although how it would effect these changes without crossing the blood-brain barrier is unclear.
Hyperforin
More recent investigations have produced evidence for hyperforin, as opposed to hypericin, as another potentially active ingredient. For example, a randomized, double-blind, placebo-controlled, 6-week study of two different extracts of SJW, with one extract containing 0.5% and the other 5% hyperforin, showed that subjects who received the SJW extract with 5% hyperforin demonstrated greater improvement in their mean Hamilton Depression (HAMD) rating scale scores than the group receiving the 0.5% hyperforin extract. The latter group showed only a slight advantage over the placebo group (9). Similar to hypericin, hyperforin may function via reuptake inhibition of serotonin, norepinephrine, and acetylcholine. Studies with isolated hyperforin and alcohol extracts of hypericum have shown that hyperforin can inhibit 5HT3/4-induced responses and uptake of serotonin (10). Animal studies conducted to investigate hyperforin’s effect on the central nervous system have revealed its antidepressant activity in the behavioral despair test. This has led investigators to hypothesize that serotonergic mechanisms are a major component in the antidepressant activities of alcohol hypericum extracts.
Hyperforin was found to enhance the extracellular levels of dopamine, norepinephrine, and serotonin, and of the excitatory amino acid glutamate (11). The levels of the main serotonin metabolite 5-hydroxyindolacetic acid, as well as those of γ-aminobutyric acid (GABA), taurine, aspartate, serine and arginine, were not influenced. These results suggest that the antidepressant property of hyperforin was due to enhanced concentrations of monoamines and glutamate in the synaptic cleft, probably as a consequence of uptake inhibition (11).
It is possible that SJW is associated with inhibition of serotonin, dopamine, norepinephrine, GABA, and L-glutamate (12), consistent with the catecholamine hypothesis of depression and more recent evidence supporting glutaminergic dysregulation in major depressive disorder (13,14). Other postulated mechanisms of hyperforin include down-regulation of cortical beta-adrenoceptors and 5-HT2-receptors, and synaptosomal release (15).
An investigation of voltage- and ligand-gated ionic conductances found that hyperforin induced a rapidly stabilizing dose- and time-dependent inward current (10). Hyperforin inhibited conductances of GABA, 2-(aminomethyl)phenylacetic acid (AMPA), and N-methyl-D-aspartate (NMDA). AMPA-induced current was competitively (though not completely) inhibited by hyperforin. NMDA receptor-activated ionic conductance was, however, uncompetitively (and completely) inhibited by hyperforin. The authors postulate a major role for hyperforin as a modulator of mechanisms involved in the control of neuronal ionic conductances, though its effects do not seem to be mediated by one single molecular mechanism of action.
Another effect of hyperforin is that it may elevate intracellular sodium concentration, hence increasing sodium-hydrogen exchange, a known mechanism of serotonin uptake inhibition. Michaelis-Menten kinetics in synaptosomes of mouse brain revealed that this type of inhibition appeared to be mainly noncompetitive (16). Low concentrations of hyperforin inhibited binding of various ligands to recombinant opioid and serotonin receptors. Hyperforin might synergistically contribute to H. perforatum‘s antidepressant effect via this mechanism (17). A reserpine-like mechanism of action for hyperforin has been proposed (18). Hyperforin (as well as hypericum extracts containing other components) inhibited tritiated serotonin accumulation in rat brain cortical synaptosomes. The inhibitory effect, however, was not due to a direct blockade of the serotonin transporters, since hyperforin did not inhibit tritiated citalopram binding. Hyperforin induced marked tritium release from superfused synaptosomes previously loaded with tritiated serotonin, suggesting a mechanism similar to the releasing effect of reserpine (18).
In contrast to the catecholamine hypothesis, one of the more interesting theories of downstream antidepressant action focuses on antidepressant-induced neurogenesis and neuroprotection (19, 20, 21). By enhancing gene expression of BDNF, VEGF, IF1, and FGF-1, all of the known antidepressants, including electroconvulsive therapy, promote neurogenesis via increased neuronal stem cell proliferation, development, differentiation, migration, and survival of cells that originate from the CA1 layer of the hippocampus in adult animal models. While the function of these new neurons is not yet known, and the exact relationship between neurogenesis and antidepressant response has not yet been fully established, recent evidence shows that these new neurons form functional synapses (22). Antidepressants also result in increased survival of existing neurons and protect them from stress-induced cytotoxic insults (20). The major putative components of SJW, hypericin and hyperforin, have actions consistent with the neurogenesis/neuroprotection hypothesis of antidepressant action (23), providing yet another plausible mechanistic rationale to pursue further careful clinical trials.
Other Components: Monoamine Oxidase-A Inhibitors
Other components of hypericum, including the flavinoids, are monoamine-oxidase-A irreversible inhibitors, but the concentration of these compounds in the extract is so small that they are unlikely to be involved in the antidepressant mechanism (24). Since the combination of selective serotonin reuptake inhibitor (SSRI) antidepressants and monoamine-oxidase inhibitors (MAOIs) should be avoided because of the danger of serotonin syndrome (25), although SJW has minimal MAOI activity it would be most prudent to avoid mixing or overlapping SJW with SSRIs.
Comment
In summary, investigations of mechanisms of action of hyperforin suggest a variety of complex actions in the central nervous system consistent with antidepressant activity. This includes uptake inhibition of serotonin, norepinephrine, and acetylcholine, antagonism at 5TH3/5HT4 receptors, antagonism at opioid receptors, stimulation of the release of serotonin, and provocation of neurogenesis.
PHARMACOKINETICS AND STABILITY
Plasma levels of hyperforin can be detected for up to 24 hours in individuals taking 300 mg coated tablets containing 14.8 mg hyperforin (26). The maximum plasma levels of hyperforin were reached 3.5 hours after administration. The plasma half-life of hyperforin was 9 hours, and its mean residence time was 12 hours. Hyperforin pharmacokinetics were linear up to 600 mg of the extract. Doses of 900 to 1200 mg of extract resulted in lower maximum drug concentration (Cmax) and area under the curve (AUC) values than those expected from linear extrapolation of data from lower doses. In a repeated dose study, there was no hyperforin accumulation in plasma. Using the observed AUC values from the repeated dose study, the estimated steady state plasma concentration of hyperforin after normal therapeutic dose was approximately 100 mg per mL (26).
EVIDENCE FOR ANTIDEPRESSANT EFFICACY
The literature as a whole suggests that hypericum has greater efficacy than placebo and equal efficacy to standard antidepressants. There are at least 37 published clinical trials, including 26 placebo-controlled studies and 14 using a standard antidepressant as the active comparator (1). Most studies have been conducted in Europe, usually in a general practice settings with patients who are already in clinical care (27). Results from such studies may be more predictive of effectiveness and acceptability in “real world” clinical practice, but are usually considered less credible than results obtained in double-blind, randomized controlled trials. In addition, the European studies generally provide limited information about recruitment methods, whether patients were recruited consecutively, and what inclusion and exclusion criteria were applied. As a further limitation, study samples in many European trials of hypericum were not limited to major depression and included other diagnoses (27). This too, may have an impact on the observed efficacy and tolerability of SJW.
SJW has been compared in clinical trials to low doses of the tricyclic antidepressants (TCAs) imipramine and maprotiline (28, 29, 30, 31). Doses of TCAs in European practice are usually lower than those considered adequate in the United States. In European trials, typical doses of imipramine and maprotiline are about 75 mg daily, though the response rates obtained appeared comparable to those in studies that use higher doses of tricyclics (e.g., imipramine >150 mg/day). The frequent lack of a placebo control makes it difficult to interpret the results, but overall hypericum appeared to be at least as effective as imipramine and maprotiline. In these studies, response rates ranged from 35.3% to 81.8% for hypericum and from 41.2% to 77.8% for TCAs.
A meta-analysis (32) examined 4 studies comprising a heterogeneous group of depressive conditions. Hypericum was administered at a dose of 300 mg three times daily, and was judged to be effective in 79 of 120 subjects (65.8%), whereas placebo was effective in only 36 of 125 subjects (28.8%; X2 32.24, P<0.0001). This placebo response rate was comparable to the typical 20% to 30% rate observed in many U.S.-based outpatient antidepressant studies.
Another meta-analysis (33) examined 15 trials comparing hypericum to placebo, and 8 trials comparing hypericum to TCAs in a total of 1,757 patients with mild-to-moderate depression. In 6 trials, hypericum outperformed placebo (55.1% response rate for hypericum, versus 22.3% for placebo) and showed comparable efficacy to TCAs (63.9% response rate for hypericum, versus 58.5% for TCAs). In two trials that used preparations of hypericum containing additional herbal medications such as Kava, SJW produced greater response rates than TCAs (67.7% versus 50%). However, a third meta-analysis (34) suggested that hypericum might not be effective for acute treatment of patients with more severe depression.
Recent Findings
Since 2001, approximately ten new studies from North America, Europe, and South America have been published. Many of these studies are noted for their large-scale, placebo-controlled, randomized, double-blind design, and/or by comparing SJW to newer antidepressants, particularly the SSRIs. In the following section, each of these studies is critiqued in detail.
St. John’s Wort vs. Standard Antidepressants
One line of research compares SJW to standard antidepressants without including a placebo arm. While some studies include too few subjects to have adequate statistical power, others have a sufficient sample to allow more decisive conclusions. Brenner et al. (35) compared SJW, type LI 160, 900 mg per day versus sertraline 75 mg per day in 30 depressed subjects for 6 weeks. Response rates were 47% for SJW and 40% for sertraline. The difference was not statistically significant, but low statistical power to detect a difference in such a small group of subjects limits confidence in the results. Gastpar et al. (36) compared SJW 612 mg per day against sertraline 50 mg per day in 241 depressed subjects for 12 weeks. 161 of these subjects received an additional 12 weeks of treatment following the acute treatment phase. In the first 12-week period, hypericum and sertraline produced comparable response rates, and subjects who continued treatment for the additional 3 months maintained their initial response. Van Gurp et al. treated 87 depressed subjects for 12 weeks in twelve community-based primary care offices, comparing SJW 900 to 1,800 mg per day to sertraline 50 to 100 mg per day (37). There were no significant differences in mean HAMD scores between the two groups, and SJW produced significantly fewer adverse events. Lack of statistical power limits the validity of the conclusions, however.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree