Static Encephalopathies and Common CNS Anomalies
Elisabeth Guthrie
Introduction
Static encephalopathy is a general term that refers to nonprogressive dysfunction of the brain. Static encephalopathy may be acquired in utero, perinatally, or postnatally. Faulty maturation of the central nervous system (CNS), prematurity, and perinatal events, as well as vascular, infectious, or traumatic insults to the CNS throughout life can all result in nonprogressive disruption of the normally functioning brain. The clinical presentation of static encephalopathy depends on the nature, timing, cerebral location, and degree of the neurological insult.
Because childhood and adolescence represent particularly dynamic periods of brain development, static deficits may change in clinical presentation and significance over time. For example, an impairment compromising visuomotor function (hand-eye coordination) may not be evident in infancy or even the preschool period. The same impairment will create significant difficulties for the school-aged child, when academic and extracurricular demands rely more heavily on fine motor coordination. Likewise, a child with a neurocognitive profile consistent with an impairment of language pragmatics or semantics, which facilitate the more nuanced aspects of communication, will encounter greater difficulties during later childhood and adolescence, as the social complexities of communication increase. The insult or injury to the brain has not changed or progressed, but the developmental context of the deficit has evolved. Some clinicians refer to these phenomena as “growing into a deficit.”
Many types of static encephalopathy are described in other chapters of this book, most notably in the chapter on intellectual disabilities. Therefore the static encephalopathy this chapter describes is limited primarily to cerebral palsy, as well as some congenital lesions involving the CNS, that a child and adolescent psychiatrist is likely to encounter in practice over time.
Certain infectious and metabolic processes involving the CNS may have an indolent course and transiently resemble static encephalopathy; however, these processes are arrested, not static, and as such are not covered here.
Cerebral Palsy
Cerebral palsy is caused by a nonprogressive insult to the developing brain that results in abnormalities of muscular movement, posture, and tone. The diagnosis of cerebral palsy in no
way infers a particular disease process or etiology, but, rather, describes a disorder of motor function that is nonprogressive, acquired in early infancy, and results in lifelong disability.
way infers a particular disease process or etiology, but, rather, describes a disorder of motor function that is nonprogressive, acquired in early infancy, and results in lifelong disability.
TABLE 11.1. GESTATIONAL AGE AND CEREBRAL PALSY | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
Cerebral palsy is the most common motor disability of childhood, with an overall prevalence of approximately 2 per 1,000 (1). The highest prevalence of cerebral palsy is among extremely low-birth-weight/extremely low-gestational-age infants (ELBW/ELGA) (Tables 11.1 and 11.2). As in many developmental disorders, male children are more commonly affected than are female children, although the reasons for this remain uncertain (2).
Despite the decline in the incidence of cerebral palsy among prematurely born infants, the prevalence of cerebral palsy has increased slightly over time (3,4). This modest increase in prevalence is attributed to the increased survival of premature infants, improved interventions for cerebral palsy over the life span that result in increased longevity, and a relatively stable rate of cerebral palsy among term births (5).
As many authors point out, it is important to underscore the fact that, despite the clear vulnerability of extremely premature low-birth-weight infants to cerebral palsy, in the vast majority of these infants, cerebral palsy does not develop (6,7).
Medical opinion regarding the etiology of cerebral palsy has changed significantly over the past two decades. Prior to the mid 1980s, the major cause of cerebral palsy was thought to be intrapartum asphyxia, with resultant hypoxic and ischemic injury to the neonatal brain. Research findings and epidemiological studies have disputed this line of causality as overly restrictive and inaccurate and point to a cascade of antenatal abnormalities and events that negatively affect placental functioning, immune mechanisms, and, ultimately, the fetal central nervous system as a more plausible mechanism resulting in the development of cerebral palsy. Many researchers conceptualize distinct differences between preterm and term infants in whom cerebral palsy develops. Gestational age and low birth weight have been studied most rigorously, but additional factors in the premature infant that compound the risk for cerebral palsy include intrauterine growth retardation (IUGR), failure to administer multiple doses of antenatal steroids during prolonged premature labor, and postnatal complications in the neonatal nursery, including respiratory distress, infection, intraventricular hemorrhage, and subsequent neonatal course (8,9).
Extreme prematurity, IUGR, and pre- and postnatal complications increase the likelihood of periventricular leukomalacia (PVL). Periventricular leukomalacia represents injury to deep cerebral white matter and is the most common pathologic correlate of cerebral
palsy. Free radicals and cytokines that are released after periods of hypoxic-ischemic stress injure the vulnerable periventricular brain tissue. Because the extremely premature infant’s brain appears to be more susceptible to injury, it sustains damage to the periventricular white matter more frequently than the brain in near-term infants (10, 11, 12). Younger fetuses are also more susceptible to intrauterine infection, as evidenced by the elevated positive amniotic fluid cultures and cytokines in women who go into labor before 34 weeks’ gestation, even in low-risk pregnancies (13). Intrauterine infection also places the infant at increased risk for PVL.
palsy. Free radicals and cytokines that are released after periods of hypoxic-ischemic stress injure the vulnerable periventricular brain tissue. Because the extremely premature infant’s brain appears to be more susceptible to injury, it sustains damage to the periventricular white matter more frequently than the brain in near-term infants (10, 11, 12). Younger fetuses are also more susceptible to intrauterine infection, as evidenced by the elevated positive amniotic fluid cultures and cytokines in women who go into labor before 34 weeks’ gestation, even in low-risk pregnancies (13). Intrauterine infection also places the infant at increased risk for PVL.
TABLE 11.2. BIRTHWEIGHT AND CEREBRAL PALSY | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
Cerebral palsy is classified according to muscle-tone characteristics (spastic, dyskinetic, ataxic, atonic) and location of motor impairment (diplegia, hemiplegia, quadriplegia). In spasticity, a consistent increase in muscle tone results in tonic muscle contraction and, over time, contractures. Dyskinesia refers to abnormal, involuntary changes in muscle tone that result in two types of movements: choreiform movements are rapid and jerky and result from sudden tonic/atonic changes in tone, whereas athetosis is a twisting, writhing motion that results from slower changes in muscle tone. A third type of cerebral palsy, ataxic, refers to abnormalities of balance and position that affect voluntary movement. (6,14). Finally, atonic, or hyptonic, cerebral palsy refers to persistent, diffuse absent or low muscle tone (14).
Spastic cerebral palsy is the most common form of cerebral palsy and represents damage to the pyramidal tracts. Spastic cerebral palsy is further classified by localization; spasticity affecting the lower extremities is referred to as diplegia; spasticity affecting ipsilateral upper and lower extremities is referred to as hemiplegia; and spasticity involving all four extremities is spastic quadriplegia (Fig. 11.1).
Spastic diplegic cerebral palsy is most commonly associated with prematurity and represents a clinical consequence of periventricular leukomalacia (1,7). As the affected child matures, the tonic contraction of large pelvic girdle and thigh muscle groups result in internal rotation and often displacement of the hip. Contraction of the thigh and
calf muscles results in flexed knees that cross the midline and tight, shortened heel cords. The resultant gait is referred to as scissoring, and the shortened heel cords result in toe walking (Fig. 11.2).
calf muscles results in flexed knees that cross the midline and tight, shortened heel cords. The resultant gait is referred to as scissoring, and the shortened heel cords result in toe walking (Fig. 11.2).
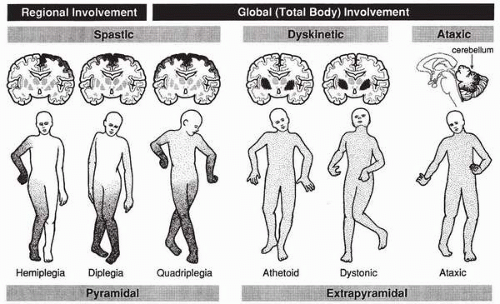 ▪ FIGURE 11.1. Different regions of the brain are affected in various forms of cerebral palsy. The darker the shading, the more severe the involvement. (Reprinted with permission from Pellegrino L. Cerebral palsy. In: Batshaw M, ed. Children with disabilities. Baltimore: Brookes Publishing, 2002:446.) |
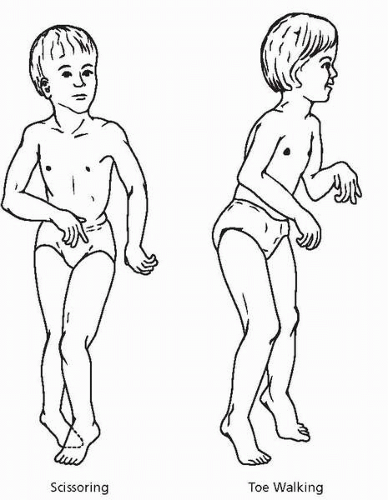 ▪ FIGURE 11.2. Scissoring results from increased tone in the muscles that control adduction and internal rotation of the hip. Toe-walking is due to an equinus position of the feet and increased extensor tone in the legs. (Reprinted with permission from Pellegrino L. Cerebral palsy. In: Batshaw M, ed. Children with disabilities. Baltimore: Brookes Publishing, 2002:451.) |
Spastic hemiplegic cerebral palsy refers to ipsilateral upper- and lower-extremity involvement, with the upper extremity usually more affected than the lower. Spastic hemiplegia is often the result of a localized vascular injury, like stroke, and, as such, is the type of spastic cerebral palsy more commonly found in term infants (1,15). Because motor function is controlled by contralateral motor neurons, a spastic left-sided hemiplegia represents a right-sided cortical injury and vice versa. A youngster with hemiplegic cerebral palsy will have a flexed upper extremity and an ipsilateral rotated lower extremity with flexion at the knee and some heel-cord shortening. The gait does not appear scissored because both lower extremities are not involved; however, the involved leg often has the appearance of being dragged as compared with the unaffected side (6).
Dyskinetic cerebral palsies involve the entire body and represent injuries to deeper, extrapyramidal brain structures. In the past, kernicterus secondary to perinatal hyperbilirubinemia was the major cause of athetoid cerebral palsy. Today, dyskinetic cerebral palsy is generally the result of abnormalities of fetal brain development or severe perinatal hypoxia and ischemia in the term infant.
Atonic, or hypotonic, cerebral palsy refers to diffuse, persistent abnormalities of absent or low muscle tone despite preserved, or even exaggerated, deep tendon reflexes. By definition,
the low tone is not secondary to peripheral nerve or muscle abnormalities. The central origins of atonic cerebral palsy are unknown (14).
the low tone is not secondary to peripheral nerve or muscle abnormalities. The central origins of atonic cerebral palsy are unknown (14).
Clinically, it is often difficult to detect cerebral palsy during the first year of life. Infants with cerebral palsy may be unable to coordinate oropharyngeal tasks, resulting in poor feeding and failure to thrive. Youngsters who have intermittent opisthonic posturing may roll over prematurely (before 2 to 3 months), and hemiplegic spasticity may lead to premature declaration of handedness (before 2 years of life), with the affected child favoring the unaffected upper extremity. A persistence of primitive reflexes and failure to meet motor milestones, especially ambulation, should alert the pediatrician to possible cerebral palsy. Some studies support developmental markers, including head control by 9 months, sitting independently by 20 months, and crawling by 30 months, to predict future ambulation. Failure to meet these developmental milestones, by 20 months for head control, 36 months for sitting, and 61 months for crawling, carries a poor prognosis for walking (16). Observations support a direct correlation between the duration of hypotonia and severity of cerebral palsy, such that those children with longer periods of hypotonia have more impairing cerebral palsy (14).
Timing in the development and the nature of the brain injury are critical factors in functional outcome. Usually, the more circumscribed the lesion responsible for cerebral palsy, the less devastating the long-term sequelae. For example, a vascular event (a stroke) in a term infant may result in hemiplegia without other sequelae. This is attributed to the observation that the infant’s brain is more adaptable and able to compensate for localized injury.
The types of deficits and challenges that confront the child with cerebral palsy depend very much on the specific type of cerebral palsy and associated impairments. Between 40% and 50% of youngsters with cerebral palsy meet criteria for an additional diagnosis of mental retardation (8,17). More than half of the youngsters with spastic cerebral palsy demonstrate cognitive and adaptive capabilities within the normal range. However, an estimated 70% of children with dyskinetic cerebral palsy have subnormal intelligence (6,14). In approximately 40% to 50% of individuals with cerebral palsy, seizures (epilepsy) develop during childhood; 35% have a seizure disorder, and 16% to 20% have severe visual impairments (8,18). In 23% of premature infants with cerebral palsy, hydrocephalus develops, contrasted with 5% of term children with cerebral palsy, a discrepancy explained by the mechanism of CNS injury (8,19).
Rates of psychiatric disorders in youngsters with cerebral palsy are described as higher than expected (20). Problems with dependency, hyperactivity, and oppositional behavior are commonly reported by parents (21). Bipolar disorder has also been reported in older teens with cerebral palsy, although, because the cases are limited to two, the significance of this finding is unclear (22). Issues of autonomy and independence in adolescence are complicated by cerebral palsy. Because more severe cerebral palsy is frequently associated with additional cognitive and sensory impairments, it is unclear how much each of these variables may contribute to the elevated rates of psychopathology. In addition, most prevalence studies of psychiatric symptoms among individuals with cerebral palsy have relied on children attending clinics at larger academic institutions. This population of youngsters with cerebral palsy may reflect a higher number of more severely affected individuals, resulting in selection bias (21). In one study of children with hemiplegia that relied primarily on parent and teacher reports and, to a lesser degree, on individual assessments, 25% of the children were found to have an “emotional” disorder, 24% had a disorder of conduct, and another 26% had some form of hyperactivity (22). On 4-year follow-up, the externalizing behaviors persisted, with hyperactivity being most predictive of continued psychiatric problems (23).
Speech and language difficulties, which are known to be associated with increased psychiatric symptoms, are common problems for children with spastic and, to a lesser degree, dyskinetic cerebral palsy. Oropharyngeal musculature is often affected in cerebral
palsy. As described earlier, when this occurs in infancy, feeding and growth difficulties commonly ensue. As an infant develops, the same musculature is recruited for speech, and other motor groups are used for nonverbal communication. Studies of speech and communication in children with cerebral palsy have described them as passive communicators who have more restricted repertoires for language communication than do their nonimpaired peers. Youngsters with cerebral palsy are less inclined to initiate conversation, answer open-ended questions, or exchange turns in a discourse with a familiar person. Not surprisingly, speech intelligibility is directly linked to these communication impairments (24). It is important that the psychiatrist be aware of these communicative constraints when working with the individual with cerebral palsy.
palsy. As described earlier, when this occurs in infancy, feeding and growth difficulties commonly ensue. As an infant develops, the same musculature is recruited for speech, and other motor groups are used for nonverbal communication. Studies of speech and communication in children with cerebral palsy have described them as passive communicators who have more restricted repertoires for language communication than do their nonimpaired peers. Youngsters with cerebral palsy are less inclined to initiate conversation, answer open-ended questions, or exchange turns in a discourse with a familiar person. Not surprisingly, speech intelligibility is directly linked to these communication impairments (24). It is important that the psychiatrist be aware of these communicative constraints when working with the individual with cerebral palsy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







