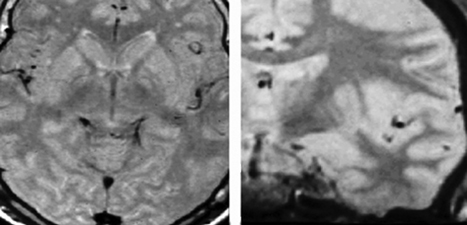
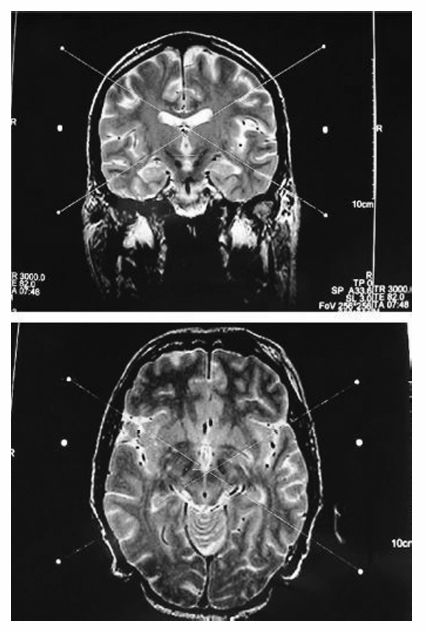
8 Stereotactic Surgery without Microelectrode Recording Marwan I. Hariz and Nathalie Vayssiere It may seem controversial to perform a functional stereotactic procedure, whether an ablation or a deep brain stimulation (DBS), without using microelectrode recording (MER). For a long time, several arguments have been presented to justify the use of MER in routine functional surgery: (1) The functional brain target could not be visualized as such on imaging studies. (2) If, however, the brain target aimed at could be visualized on magnetic resonance imaging (MRI), there was still concern about distortions in the image. (3) If brain atlas and ventricular landmarks—the anterior commissure (AC), posterior commissure (PC), and midline of the third ventricle—were used to indirectly determine the anatomical location of the brain target, there was concern about interindividual anatomical variabilities. (4) One could never be sure of the accuracy of the stereotactic frame and the probes used. (5) Functional stereotactic surgery is a physiological more than an anatomical procedure. Rather than debating these arguments,1,2 this chapter outlines alternatives to MER for functional stereotactic procedures. A non-MER-guided technique must obviously rely on other means than MER to achieve the success and safety of the procedure. These means may include (1) an adequate and validated preoperative stereotactic MRI method designed to visualize the individual target in the individual patient using appropriate scanning sequences to allow direct targeting without relying on brain atlas and ventricular landmarks; (2) a geometrically and mechanically accurate frame that is compatible with the imaging method used; (3) a radiofrequency (RF) machine to monitor the impedance along the track from the cortex to the target using a straight electrode with a noninsulated tip of no more than 2 mm in diameter or length; (4) a macrostimulation using standardized pulse width, frequency, and current intensity; (5) knowledge of the macroelectrophysiological response and of the functional anatomy of the target and its surrounding area so as to know where to move the electrode next in case of nonadequate response to stimulation in the awake, nonsedated patient; (6) immediate postoperative imaging performed in stereotactic conditions, with scanning sequences adapted to visualize with minimal artifacts both the target and the implanted DBS lead. This chapter discusses these issues and their pros and cons as well as the variations in imaging, impedance, and macrostimulation parameters, according to brain target [i.e., the ventral intermediate (Vim) nucleus of the thalamus, the posteroventral pallidum (PVP), or the subthalamic nucleus (STN)]. Two aspects have to be considered for MRI to be suitable as a source for direct determination of coordinates in functional neurosurgery. First, it must be able to provide good discrimination of the targeted brain structures (good structural definition); and second, the geometrical accuracy of the MRI scan has to be validated. General requirements to perform stereotactic imaging may include the following: (1) a stereotactic frame with an MRI localizer that is rigid (no possibility of deformation, or strain), and with good MRI compatibility (visibility on MRI, sensibility on image deformation, with a size as small as possible to allow for fiducials to be as close as possible to the head); (2) a suitable adapter to fix the frame in the MRI unit; (3) an adapted MRI sequence with ability to provide a good discrimination of targeted brain structures (good structure definition, see later discussion); (4) a field of view that fits the stereotactic frame; (5) a slice thickness allowing good compromise between resolution (the smallest) and signal:noise ratio (1.5 to 2.0 mm thickness is a good compromise); (6) isometric pixel size in the axial plane (x,y; square matrix, scan percentage of 100%); (7) enough slices to cover the target area with good margins, preferably without a gap between slices, and no overcontiguous slices (to avoid an interpolation of the signal between two slices); (8) a head coil to ensure maximal signal; (9) a protocol to minimize distortion through a regular check of the gradient fields (rigorous maintenance protocol); (10) control of movement during acquisition (general anesthesia if necessary); (11) weekly refills of the localizer box with new liquid (CuSO4). With few exceptions,3,4 most functional stereotactic surgeons today do use a stereotactic MRI study to determine target coordinates. However, many workers who indeed use MRI still relate the target to its position in relation to visualized ventricular landmarks on the MRI study.5–9 Unlike ventriculography and CT scanning, MRI is not a homogeneous imaging method. Depending on the parameters of imaging, an MRI study can visualize differently and unequally well various structures in the brain. The ventral intermediate nucleus (Vim) of the thalamus is the only brain target in movement disorder surgery that still cannot be visualized as such on stereotactic thin slice MRI. Nonetheless, MRI, especially a T2-weighted MRI sequence with thicker slices, does indeed visualize the thalamocapsular border, making it easier to determine at least the laterality of the Vim target. Currently, the Vim target is used for essential tremor. The other commonly used targets in surgery for Parkinson disease and dystonia are the posteroventral pallidum and the STN. These structures can be exquisitely visualized on MRI, provided proper scanning sequences are used. The subdivisions of the globus pallidus [globus pallidus pars interna (GPi), laminae medullaris interna and externa, globus pallidus pars externus (GPe)] and their surrounding structures (putamen, internal capsule, optic tract) can be visualized stereotactically on thin-slice axial and coronal MRI using various sequences. One such sequence10 is a nonvolumetric proton density sequence (repetition time (TR)/ time to echo (TE) 4000/15, echo-train 7, field of view 250 mm, slice thickness 2 mm, gap 0, matrix 210 × 256, excitations 3, imaging time 6 min, 5 sec) that depicts exquisitely the details of the pallidal target area (Fig. 8.1). Another sequence that has been validated by VayssiËre uses volumetric T1 sequences.11,12 Other MRI scanning methods, based on inversion recovery sequences, have been described by Starr et al.13 In all these cases, because the target itself is readily visualized, there is no need to refer to the landmarks of the third ventricle and to an atlas to obtain the location of the target in the individual patient, especially since it has been shown that the individual target location may vary substantially between patients, and also between the two hemispheres in the same patient.10,13 In surgery on the STN, most workers who rely on MRI for targeting this structure determine its position on T1-weighted images in relation to third ventricle landmarks and brain atlases. The few publications reporting on the use of stereotactic MRI for direct visualization and targeting of the STN describe volumetric T2-weighted sequences with a rather long acquisition time,14–18 sometimes requiring reformatting of images or additional T1-weighted sequences that are used for targeting, and often necessitating general anesthesia during imaging. These sequences do allow exquisite visualization of the STN. The present author, together with others,19 has implemented a nonvolumetric T2-weighted MRI sequence (TR 3000–4000, TE 80–100) allowing individual visualization of the STN with fast acquisition sequences (between 3 min 5 sec and 7 min 48 sec, depending on the MRI machine) (Fig. 8.2). Here also, direct visualization made it possible to target the center of the visualized STN at surgery, avoiding an indirect localization based on the atlas and the AC—PC landmarks. In all these imaging procedures, the authors have used either or both the Laitinen stereotactic apparatus19–21 and the Leksell Stereotactic System (Elekta AB, Stockholm, Sweden),11,12,22 together with MRI machines of various makes (Siemens, Philips, General Electric), that have undergone regular inspections for field inhomogeneity and other sources of distortions. Three methods can be used for physiological corroboration of the anatomical target in nonmicroelectrode-guided surgery: impedance recording, mechanical stunning effect when the probe reaches the target, and macrostimulation. Fig. 8.1 Axial and coronal stereotactic magnetic resonance imaging showing the details of the posteroventral pallidum and its surrounding structures. Fig. 8.2 Axial and coronal stereotactic magnetic resonance imaging showing the subthalamic nucleus. (See Color Plate 8.2.) For impedance recording a radiofrequency (RF) electrode and RF generator are needed. Measurement of brain tissue impedance (resistance) to electrical current between a small probe tip and a large indifferent electrode is an old and excellent method to locate different structures inside the brain.23 If the tip of the measuring probe is very small in surface area in relation to a large indifferent electrode, the impedance refers to the immediate vicinity of the probe tip. When the stereotactic probe is introduced toward the target area, the impedance is followed on a panel meter and by audio monitoring. Just by listening to the impedance pitch, the surgeon can detect the probe’s location within a given structure. The Wheatstone bridge used for the impedance measurement gives the most accurate results in the brain, when the oscillator frequency is 1 to 10 kHz. Then the impedance of the white matter is ~25% higher than that of the gray matter. The smaller the uninsulated probe tip, the more local-specific is the impedance recording. Therefore, uninsulated tips of the order of 1 to 2 mm in length and diameter are recommended. When the probe comes from the white matter perpendicularly into the gray matter of the putamen and pallidum, as for instance during pallidal procedures, the impedance fall is clearly noticed within 1 mm between white matter tracts and the anterodorsal putamen or anterodorsal pallidum, depending on angulation of the electrode trajectory. Typically, a white matter impedance of 600 to 800 ohm (depending on thickness of electrode) falls to 400 to 500 ohm into the gray matter. As the probe then approaches the base of the pallidum and the cistern between the pallidum and the temporal lobe, the impedance rapidly falls to 150 to 250 ohms. If the trajectory is such that the electrode exits the medial edge of the pallidum into the internal capsule, the impedance becomes suddenly higher again. In cases where an electrode tip of 1.0 to 1.5 mm is used, one can sometimes notice, just by listening to the impedance, when the electrode coming from the GPe toward the base of the globus pallidus internus (GPi), traverses the white matter of the internal medullary laminae. During thalamic procedures, with a frontal approach, the probe penetrates the caudate nucleus with a low, gray matter impedance. In the white matter of the internal capsule the impedance rises again then falls again slowly toward the thalamus. As the probe comes to the vicinity of the subthalamic white matter the impedance starts rising again. The less sharp profile of impedance during ventrolateral thalamic surgery (compared with that during pallidal surgery) is due to the fact that the probe advances rather parallel to the thalamocapsular border. When the probe is targeting the STN, one can notice, depending on angulation and trajectory of electrode toward the STN, first a rise of impedance at the immediate subthalamic area, then a decrease of impedance upon entering the STN; the impedance values here are, however, less distinctive due to the fact that the zona incerta contains small areas of mixed white matter and gray matter. If on any trajectory the probe traverses the ventricle or any cistern or cystic cavity, there is a sharp decrease of impedance. Fig. 8.3B shows the impedance values in a case of pallidal surgery where the electrode (2 mm thick) ended up in capsular white matter. It was subsequently repositioned to end up in pallidal gray matter (Fig. 8.3A). Intraoperative stimulation at both locations confirmed in this patient the location of the electrode (see the macrostimulation section). Fig. 8.3C shows a cerebrospinal fluid (CSF) impedance value in a case where the electrode traverses a ventricular space. Hence, the impedance measurement, which does not require any extra time, provides an excellent method with which to check whether the probe is in gray matter, white matter, or CSF space. Fig. 8.3 Impedance values in (A) gray matter, (B) white matter, and (C) cerebrospinal fluid measured with a 2 mm thick radiofrequency electrode. (See Color Plate 8.3.) It is clinically important to observe carefully what may happen when the stereotactic probe comes to the vicinity of the surgical target. In surgery on the Vim thalamus and the STN, this mechanical effect may be very evident: In Vim surgery, tremor can sometimes be abolished just by a stunning effect when the electrode reaches the target. This stunning effect may last from a few seconds to several days. In surgery on the STN, sometimes the mere introduction of the DBS electrode provokes a sudden decrease in rigidity (assessed at the level of the wrist) and appearance of dyskinesias. The mechanical effect, also called microlesion (microthalamotomy or microsubthalamotomy) is a very good sign during surgery, suggesting accuracy in reaching the target. It may, however, not always be predictive of good and sustained long-term effect. Contrary to what some believe, this microlesioning effect does not preclude conducting a macrostimulation of the target because, even if there are no more symptoms to stimulate (i.e., tremor or rigidity), stimulation can still be used to evaluate side-effects (see later discussion). In the pallidum, rarely do symptoms decrease by the mere introduction of the electrode to the target. As for impedance recording, it is also important for stimulation that the probe tip is small. This guarantees that the stimulation effects are local-specific. If the uninsulated tip of the electrode is longer, for instance 4 or 5 mm, one cannot know which part of the tip generates the stimulation response. When stimulation is conducted using an RF electrode, most stereotactic surgeons use rectangular, biphasic waves, 0.2 to 1.0 millisecondsec in length. Even though some RF generators are still based on constant voltage, it is preferable to use constant current stimulators, where the current intensity is independent of tissue impedance change. In case the surgery is a DBS and not a lesional procedure, it is preferable to use the DBS lead itself for intraoperative stimulation because this is the lead that will subsequently be used for chronic stimulation; besides, one has ready access to four different contacts allowing a mapping of the stimulation response in the target area along the track of the DBS lead within the target. Stimulation can be performed in a monopolar or bipolar fashion or both. In general, bipolar stimulation is more local-specific than monopolar but may require higher intensities and a larger pulse width. Typically, a 60 or 90 μsec pulse width is used, with a frequency of 130 Hz or more. The current intensity used varies from tissue to tissue. Depending on the nature of the brain target, intensities of 0.5 V up to 5 V can be used (see later discussion). Electrical stimulation together with observation of the mechanical introduction effect and impedance recording makes the stereotactic surgery relatively safe. If needed, the probe can be repositioned. Then the stimulation procedure is repeated. It must be stressed, however, that at the repositioning of the probe, the mechanical introduction characteristics as well as the impedance values and stimulation responses may have been modified by the first insertion of the probe. This is a drawback of macrostimulation-guided surgery. During each intraoperative stimulation (and RF coagulation) it is important to check the patient with respect to the following: alertness, orientation, memory, speech articulation and voice strength, facial expression, limb strength, limb movements, limb dexterity, limb coordination, sensations (at fingertips, cheek, tongue and lips—paresthesias), vision and eye movements, eventual autonomic responses (sweating, heart rate, etc.), and of course eventual effect of stimulation on tremor, rigidity, akinesia, dyskinesias, and so forth. One should also maintain a conversation with the patient and ask the patient to report feeling anything special. Asking the patient to count backward or list weekdays or months backward will generate a small amount of stress, which will elicit or enhance symptoms and allow better assessment of the effect of stimulation on these symptoms. A record should be kept of the stimulation parameters and the patient’s reactions during surgery. Too low a stimulation threshold before effect or side effect (< 0.5 V, 130 Hz, 1 millisecondec) may indicate the position in the internal capsule or sensory thalamus. Even if tremor is decreased or arrested, a capsular location of the electrode may at lower voltages provoke cramps and increased difficulties in opening or closing the hand. Also, dysarthria or even speech arrest may be induced (especially during left-sided stimulation). At higher voltages, a tetanic cramp of the hand or lips may be elicited, as well as nausea. In the sensory thalamus, paresthesias at the fingertips or lips may be elicited. These features may urge repositioning of the probe in the medial or anterior direction, respectively. A too high stimulation threshold before obtaining a tremordecreasing effect (> 3 V, 130 Hz) indicates the probe’s position is too anterior, too medial, or too dorsal. If thalamic high-frequency stimulation yields electrical feeling in the depth of the fingers, hand, or arm, and tremor arrest, with concomitant precise, fluid, and strong movements, good coordination, and good speech, then the probe must be in a good position. One can stress the patient by asking the patient to count backward to see if the tremor recurs. The same testing protocol can be applied to stimulation through the other DBS contacts to verify how many contacts would be effective for the symptoms and at what intensities, and with what side-effect profile. Intraoperative stimulation should always be conducted with rather large suprathreshold values to verify the safety window between stimulation values effective for tremor and values yielding side effects. As discussed earlier, if the mere introduction of the electrode stops the tremor, stimulation should indeed be done to detect side effects prior to permanent implantation. If stimulation responses are not adequate, the position of the electrode has to be changed: After appropriately changing the coordinates on the frame, the electrode should be reintroduced toward the new target through a new cortical entry point within the context of the 14 mm burr hole, and the whole stimulation procedure should be repeated. In the authors’ opinion, this may be done up to three times maximally, after which, if the response to stimulation is still poor, the surgery should be aborted. The stimulation thresholds in PVP are higher than those in the thalamus. Furthermore, the main aim of intraoperative stimulation here is not primarily to provoke an arrest of the symptoms (like tremor arrest during thalamic stimulation) but to avoid the internal capsule and optic tract. The effects of acute pallidal stimulation on the parkinsonian symptoms per se are not consistent unless one applies the stimulation for a long time (several minutes), which may not be practical during surgery. In routine intraoperative pallidal stimulation, however, sometimes nothing happens, sometimes stimulation increases the speed of movement of the leg or hand, and sometimes, more rarely, dyskinesias are elicited. This paucity of reaction in the pallidum is, however, not the case when a lesion is being performed, because, during the staged lesioning, there is almost invariably a release of rigidity and a striking amelioration of finger, hand, and leg dexterity. To maximize the symptoms for the time of surgery, all parkinsonian medications should be stopped at least 6 hours before the operation. The burr hole should be 2.0 to 2.5 cm from the midline at the level of or slightly anterior to the coronal suture. Electrical stimulation, using a rigid RF electrode, is carried on with 5 to 6 Hz up to 8 to 10 mA and 130 Hz up to 4 to 5 mA, respectively. If, at these current intensities, stimulation does not give rise to any undesirable reactions (capsular or optic), then a coagulation is safe (if one is doing a lesional surgery) or the permanent DBS electrode can be implanted at this site, and stimulation is conducted in the same way as for thalamic surgery as far as observing the patientás reactions is concerned, but with higher thresholds to detect side effects. It must be stressed that, at least in Europe, pallidal DBS is used much more for dystonia surgery than for PD surgery. Dystonia patients are most often operated on under general anesthesia,24 which may preclude the use of stimulation as a means of localizing the position of the electrode. Here it is mandatory to perform an immediate postoperative stereotactic MRI to verify the exact location of the leads (see later discussion). At a fixed frequency of 130 Hz and pulse width of 60 μsec, the following can be obtained with monopolar stimulation of the STN through a DBS lead contact: a sudden decrease of rigidity assessed at the wrist (signe de Pollak-Limousin), a decrease or arrest of tremor, a gradual improvement of hand opening and finger dexterity, and sometimes the appearance of dyskinesias, starting typically on the contralateral foot. These responses may be obtained at amplitudes of 0.5 to 3.0 V. If none of these responses is obtained at these voltages, the probe is probably not in the STN. By increasing the voltage, one can obtain side effects that can be used to guide movement of the probe. If increased stimulation results in cramping in the hand or face together with dysarthria, the probe is probably in capsular peduncular white matter and should be moved medially and/or anteriorly or posteriorly. If paresthesias are elicited, the probe is too posterior or too medial or both. If there is a convergent eye deviation or pupil dilatation, the probe is too medial, affecting the oculomotor fibers. If there is an increase in tremor and rigidity, the probe is too close to or into the red nucleus. If there is increased rigidity associated with mood changes, the probe is probably too deep, affecting the substantia nigra. In the STN and pallidum, it is advantageous to perform macrostimulation using the permanent DBS lead because this lead has four contacts that can be stimulated monopolarly one by one, allowing macromapping of the area along the track of the electrode. Another advantage is that the parameters of stimulation are close to those that will be ultimately used in chronic stimulation. Finally, by using the DBS lead for intraoperative macrostimulation, there is no need for replacing it once one is happy with the stimulation response: it just has to be secured to the burr hole at the level at which maximum benefit and lowest side effects are obtained. In 1982, Tasker, Organ, and Hawrylyshyn published a book about the use of macro-stimulation in mapping the thalamus and midbrain in humans. In this 500-page book, entitled The Thalamus and Midbrain of Man: A Physiological Atlas Using Electrical Stimulation,58 the authors provided a detailed atlas about the gross somatotopy of the subdivisions of the thalamus and midbrain, based solely on intraoperative macrostimulation. Furthermore, in a chapter published in Neurosurgery Clinics of North America in 1990 (pp. 846–847),31 Tasker, who is a scientist and neurosurgeon with broad experience in both macroelectrode and MER, compared both techniques and stated the following: Macrostimulation is easy, requires minimal instrumentation, is quick, identifies a wide range of brain structures, even at variable distances from probe…. Microelectrode techniques are more difficult, time consuming, require more sophisticated equipment …. Microelectrode can identify only a limited repertoire of structures. The tip must be very close to a structure before it can be recognized at all…. The more limited current spread means that unless an excitable structure is very close, it will be missed; the surgeon may gain no clue from a “negative” trajectory where to seek next. Thus macrostimulation is indeed a physiological method, simple to use, and readily available. The drawback of this technique is that sometimes it may be difficult to interpret the findings and to know in which direction one has to relocate the probe in case of unsatisfactory stimulation response, and in case no readily understandable structure, such as the sensory thalamus, the internal capsule, or the optic tract, is encountered upon macrostimulation. In surgery on the Vim during which the probe misses the Vim, unless the electrode is laterally or posteriorly misplaced, stimulation may not give a clue as to which direction the probe should be moved. Is the probe too medial or too anterior? The same applies if, in pallidal surgery, the probe is too lateral or too anterior. The most difficult to interpret are the stimulation results in the area of the STN if the STN proper is missed upon the first introduction of the electrode. Furthermore, repeat introductions of macroelectrodes in view of finding the target may alter the physiology and anatomy of the target area, and one is lost as to what is being stimulated and how to interpret the response. In such cases, and after two to three introductions of the probe without stimulation benefit, either the surgery has to be interrupted or an intraoperative stereotactic MRI has to be performed before carrying on additional multiple penetrations to evaluate the location of the probe. This is especially the case if the STN has been missed at the first or second introduction. Hence the importance of postoperative stereotactic imaging in non-MER-guided surgery. In patients who receive DBS in general anesthesia such as patients with generalized dystonia, postoperative stereotactic MRI is simply mandatory. In these cases, no macrostimulation is possible, and even if these patients are operated on in local anesthesia, intraoperative stimulation may show nothing in terms of relief of symptoms. Therefore, one may argue that in dystonia patients MER is mandatory. However, some of the best reported results have been published by the team of Philippe AndrÉ Coubes in Montpellier, who performs pallidal DBS in general anesthesia for pediatric and adult dystonia, with neither recording nor stimulation, but using only a rigorous stereotactic imaging technique, both preand postoperatively.24,59 Stereotactic postoperative MRI is important in that it will show nonequivocally the exact location of the stereotactic lesion or the DBS electrode. This imaging should be done with the same parameters as the preoperative imaging. In cases of ablative surgery, however, it is an advantage that postoperative imaging be done several weeks after surgery to allow for postoperative edema to resolve. Unless a noninvasive frame such as the Laitinen frame has been used during surgery and repositioned on the head for the postoperative imaging,60 the scanning can still be done with thin (2 mm thick) axial slices parallel to the AC—PC line, and thin coronal slices perpendicular to the AC—PC line. T1 or proton density sequences may be used, sometimes even T2 or inversion recovery, allowing visualization of the lesion, the target, and its boundaries (Fig. 8.4). In patients with DBS, postoperative imaging should be performed immediately after surgery while the frame is still on the head. If the neuropacemaker has already been implanted, MRI can still be done provided the voltage of the pacemaker is set to zero and the output is off before the patient enters the MRI room. Here also, one should be able to assess the location of the lead within the visible target (Fig. 8.5), and if necessary, return the patient to the operating room to relocate the lead should it become misplaced.
Stereotactic Imaging
General Requirements
Ventral Intermediate Nucleus of the Thalamus
Posteroventral Pallidum
Subthalamic Nucleus
Electrophysiological Testing and Intraoperative Target Adjustment
Impedance Recording

Mechanical Introduction Effect
Macrostimulation
Macrostimulation in Vim
Macrostimulation in the Posteroventral Pallidum
Macrostimulation in the Subthalamic Nucleus
Advantages and Disadvantages of Surgery without Microelectrode Recording
Postoperative Stereotactic Magnetic Resonance Imaging
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








