Chapter 2 Stroke
Introduction – time is brain
According to The Stroke Association (2010), every 5 minutes, someone in the UK has a stroke. This means that in Great Britain alone, approximately 150,000 people have a stroke every year. Stroke is the third biggest cause of death and the biggest cause of adult disability.
A stroke is a medical emergency and anyone suspected of having a stroke should be taken to Accident & Emergency immediately. The UK Stroke Association aims to raise stroke awareness and has organized the FAST campaign (Figure 2.1). FAST is an acronym standing for Face, Arm, Speech, Time to call 999. When you suspect someone is having a stroke, test facial weakness (can the person smile?), arm weakness (can the person raise both arms?) and speech problems (can the person speak clearly and understand what you say?). If the answer to any of these questions is no, the person might have a stroke so it is time to call 999 – because stroke is a medical emergency.
Making a prognosis directly after stroke is difficult and depends on a variety of factors, which will be presented later in this chapter. Overall, approximately 20% of patients having their first stroke are dead within a month, and of those alive at 6 months approximately one-third are dependent on others for activities of daily living (Warlow, 1998).
Definitions
A stroke or cerebrovascular accident (CVA) is typically defined as an accident with ‘rapidly developing clinical signs of focal or global disturbance of cerebral function, with symptoms lasting 24 hours or longer or leading to death, with no apparent cause other than of vascular origin’ (WHO, 1988).
Classification and aetiology of stroke
Strokes are classified into two main categories: ischaemic or haemorrhagic (Amarenco et al., 2009). An ischaemic stroke is caused by an interruption of the blood supply. A haemorrhagic stroke is caused by a ruptured blood vessel. The majority of strokes are ischaemic accidents (approximately 80%).
The main causes of ischaemic stroke are:
Strokes are thus typically classified as ischaemic or haemorrhagic. Ischaemic strokes are commonly further classified according to the Oxford Community Stroke Project (OCSP) classification, also known as the Oxford or Bamford classification (Bamford et al., 1991). This classification distinguishes between a:
Anatomy and pathophysiology
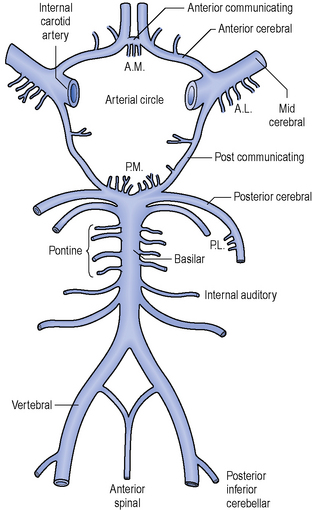
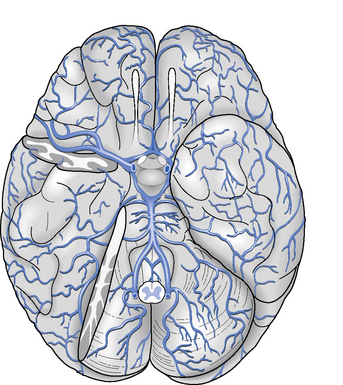
The arteries that supply blood to the brain are arranged in a circle called the Circle of Willis (Figure 2.2), after Thomas Willis (1621–1673), an English physician. All the principal arteries of the Circle of Willis give origin to secondary vessels which supply blood to the different areas of the brain (Figure 2.3).
When an ischaemic stroke occurs and part of the brain suffers from lack of blood, the ischaemic cascade starts. Without blood the brain tissue is no longer supplied with oxygen and after a few hours in this situation, irreversible injury could possibly lead to tissue death. Because of the organization of the Circle of Willis, collateral circulation is possible, so there is a continuum of possible severity. Part of the brain tissue may die immediately while other parts are potentially only injured and could recover. The area of the brain where tissue might recover is called the penumbra. Ischaemia triggers pathophysiological processes which result in cellular injury and death, such as the release of glutamate or the production of oxygen free radicals. Neuroscience research is constantly studying ways to inhibit these pathophysiological processes by means of developing neuroprotective agents (Ginsberg, 2008).
Diagnosis
The diagnosis of stroke is based on a clinical assessment and imaging techniques such as computed tomography (CT) or magnetic resonance imaging (MRI) scans. For diagnosing an ischaemic stroke in the acute setting, an MRI scan is preferred, as sensitivity and specificity are higher in comparison with CT imaging (Chalela et al., 2007). For diagnosing ischaemic strokes, CT and MRI scan have comparable sensitivity and specificity.
Early medical treatment
In the case of an ischaemic stroke, the more rapidly the blood flow is restored to the brain, the fewer brain cells die (Saver, 2006). Hyperacute stroke treatment is aimed at breaking down the blood clot by means of medication (thrombolysis) or mechanically removing the blood clot (thrombectomy). Other acute treatments focus on minimizing enlargement of the clot or preventing new clots from forming by means of medication such as aspirin, clopidogrel or dipyridamole. Furthermore, blood sugar levels should be controlled and the patient should be supplied with adequate oxygen and intravenous fluids.
Thrombolysis is performed with the drug tissue plasminogen activator (tPA); however, its use in acute stroke is controversial. It is a recommended treatment within 3 hours of onset of symptoms as long as there are no contraindications, such as high blood pressure or recent surgery. tPA improves the chance of a good neurological outcome (The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group, 1995). In a recent study, thrombolysis has been found beneficial even when administered 3 to 4.5 hours after stroke onset (The European Cooperative Acute Stroke Study, 2008). However, another recent study showed mortality to be higher among patients receiving tPA versus those who did not (Dubinsky & Lai, 2006).
Another intervention for acute ischaemic stroke is the mechanical removal of the blood clot. This is done by inserting a catheter into the femoral artery, which is then directed into the cerebral circulation next to the thrombus. The clot is then entrapped by the device and withdrawn from the body. Studies have shown beneficial effects of thrombectomy in restoring the blood flow in patients where thrombolysis was contraindicated or not effective (Flint et al., 2007).
Prognosis and recovery
Van Peppen and colleagues (2007) have performed a systematic review of prognostic factors of functional recovery after stroke. They investigated walking ability, activities of daily living, and hand and arm use after stroke.
Walking ability (defined as a Functional Ambulation Category (Holden et al., 1984) score ≥4) at 6 months after stroke was best predicted by initial walking ability in the first 2 weeks after stroke, degree of motor paresis of the paretic leg, homonymous hemianopia, sitting balance, urinary incontinence, older age and initial ADL functioning in the first 2 weeks after stroke (Kwakkel et al., 1996).
The Barthel Index score (Mahoney & Barthel, 1965) in the first 2 weeks after stroke appeared to be the best prognostic factor for recovery of independence in activities of daily living at 6 months after stroke. Other contributing predictors were urinary incontinence in the first 2 weeks after stroke, level of consciousness in the first 48 hours after stroke, older age, status following recurrent stroke, degree of motor paresis, sitting balance in the first 2 weeks after stroke, orientation in time and place, and level of perceived social support (Kwakkel et al.,1996; Meijer et al., 2003).
The best clinical predictor of recovery of dexterity of the paretic arm 6 months after stroke appeared to be severity of arm paresis at 4 weeks after stroke, measured by Fugl-Meyer Arm Assessment (Kwakkel et al., 2003). Other studies also identified severity of the upper extremity paresis, voluntary grip function of the hemiplegic arm, voluntary extension movements of the hemiplegic wrist and fingers within the first 4 weeks after stroke, and muscle strength of the paretic leg (Heller et al., 1987; Kwakkel et al., 2003; Sunderland et al.,1989).
Hendricks et al. (2002) conducted a systematic review of the literature of motor recovery after stroke. They concluded that approximately 65% of the hospitalized stroke survivors with initial motor deficits of the lower extremity showed some degree of motor recovery. For patients with paralysis, complete motor recovery occurred in less than 15% of cases, both for the upper and lower extremities. The recovery period in patients with severe stroke appeared twice as long as in patients with mild stroke.
There are several studies indicating that most of the overall improvement in motor function occurs within the first month after stroke, although some degree of motor recovery can continue in patients for up to 6 months after stroke. Verheyden et al. (2008) compared the recovery pattern of trunk, arm, leg and functional abilities in people after ischaemic stroke. They assessed participants at 1 week, 1 month, and 3 and 6 months after stroke. There appeared to be no difference in the recovery pattern of trunk, arm, leg and functional ability and, for all measurements, most (significant) improvement was noted between 1 week and 1 month after stroke. There was still a significant improvement between 1 month and 3 months after stroke, but between 3 and 6 months participants showed no more significant improvement. Further exploration of this latter period saw some participants stagnate in trunk, arm, leg and functional recovery and others deteriorate. Deterioration in people after stroke has been demonstrated in other (long-term) studies (van de Port et al., 2006). But despite evidence of stagnation, deterioration or a plateau phase, there is substantial secondary evidence concerning late recovery, i.e. several months after stroke, although most of these studies were in (outpatient) rehabilitation centres and thus included selected patient populations. Nevertheless, Demain et al. (2006) suggested that the notion ‘plateau’ is conceptually more complex than previously considered and that ‘plateau’ not only relates to the patients’ physical potential, but is also influenced by how recovery is measured, the intensity and type of therapy, patients’ actions and motivations, therapist values and service limitations.
Outcome measures
Milestones of stroke rehabilitation should be documented by means of standardized outcome measures (Stokes, 2009). There is an increasing number of tools available. Van Peppen et al. (2007) have performed a systematic review of outcome measures for people with stroke. They propose a core set of outcome tools based on consistency with the International Classification of Functioning, Disability and Health (WHO, 2001); high-level psychometric properties (i.e. inter- and intrarater reliability, validity and responsiveness); good clinical utility (easy and quick to administer); minimal overlap of the measures and consistency with current physiotherapy practice. The core outcome measures proposed for people with stroke based on their review were:
Van Peppen et al. (2007) also propose a set of 18 optional outcome measures, to be used to evaluate a specific function or activity in people with stroke. These optional outcome measures are:
Clinical utility of an outcome measure is probably a key aspect and often authors have neglected this area in the past. A recent study by Tyson and Connell (2009) looked at how to measure balance in clinical practice. They performed a systematic review of measures of balance activity for neurological conditions. They scored not only psychometric properties, but also clinical utility by assigning scores to the time taken to administer, analyse and interpret the test, the costs of the tool, whether the measure needs specialist equipment and training, and whether the measurement tool is portable. They evaluated 30 measures and after excluding 11 based on limited psychometric analysis or inappropriate statistical tests used, they recommended the following balance tools for people with stroke:
Principles of physical management
Time course
The National Clinical Guidelines for Stroke (Intercollegiate Working Party for Stroke, 2008) and the National Strategy for Stroke (Department of Health, 2007) include recommendations that people who have had a stroke are ideally managed in a stroke unit by a specialist multidisciplinary team. Even so, there are different types of clinical services, pathways and, indeed, stroke units throughout the country with varying criteria for admission and discharge. Organized services with specialist multidisciplinary teams have been identified as key to a positive outcome. A physiotherapist should expect to work with other health-care staff dedicated to the care of people with stroke and to contribute to the process of problem solving and decision making involved in the overall management. Although stroke rehabilitation commences in the acute stage on admission to hospital, active participation in the relearning of mobility and independence broadly takes place during the sub-acute and long-term stages post stroke. These three stages are rarely distinctive, they frequently overlap and do not always follow the same time frame or order for everyone, but there are common patterns. In addition, the process of transferring from hospital or rehabilitation setting and discharge from services requires structured management and should be carefully planned to be effective.
Acute stage
Patients in the acute stage can be at different levels of consciousness; they may be sedated and intubated (Kilbride & Cassidy, 2009a) or they may be able to communicate with or without difficulty. It is essential to find out if the person is medically stable before commencing physiotherapy treatment; talk to key members of the hospital team and read the medical notes. Find out the age of the patient, the type of stroke, blood pressure, ability to communicate, if an injury occurred at the time of their stroke and information about their medical history, for example have they experienced a previous stroke or do they have dementia? An outline of their social environment may also provide a guide on cultural issues and language differences. It is important to be informed of those potential risk factors that may influence what a physiotherapist does and the treatment planned. It is essential to report to nursing staff and therapists before commencing treatment. Assume all patients understand you even though they may not appear to. At a later stage, many patients describe conversations overheard between members of staff who were either talking about them or ignoring them in the early period. Remember a stroke is an event that happens suddenly; the previous day your patient could have been a director of a company, a highly skilled worker, or an independent active mother or grandmother. The change in situation can be frightening and a shock. If members of the family are present when you visit, they are also likely to be shocked and confused. Introduce yourself and say what you will be doing.
The emphasis during the early days is on ensuring normal respiratory function, skin care and management of mobility; initially this may comprise positioning or passive movements to ensure the maintenance of the length of soft tissue and range of movement, particularly when muscles work over more than one joint (Kilbride & Cassidy, 2009a). All people with stroke impairments after 24 hours should receive a full multidisciplinary assessment using an agreed procedure within five working days and this should be documented in the notes (Intercollegiate Working Party for Stroke, 2008).
Passive and active mobilizing can be assessed in the early stages in more than one position when the condition permits, for example lying, side lying and sitting. Through observation and handling during the process of moving the patient into the different positions, the physiotherapist will be able to judge the amount of impairment an individual has, their ability to control movement in particular head and trunk posture and their ability to initiate movement and to follow commands. Control of head and trunk position in the upright posture in the first few days is a positive indicator of future functional independence (Intercollegiate Working Party for Stroke, 2008).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree