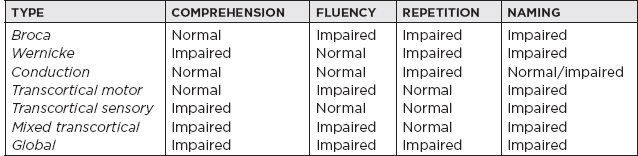
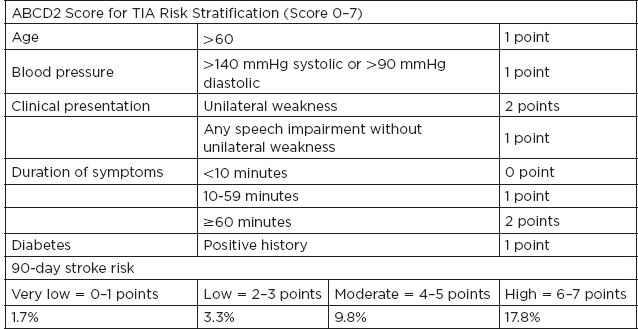
I. Definitions A. Ischemic stroke: an episode of neurological dysfunction caused by focal cerebral, spinal, or retinal infarction (clinical syndrome) B. Central nervous system (CNS) infarction: brain, spinal cord, or retinal cell death attributable to ischemia, based on: 1. Pathological, imaging, or other objective evidence of cerebral, spinal cord, or retinal focal ischemic injury in a defined vascular distribution; or 2. Clinical evidence of cerebral, spinal cord, or retinal focal ischemic injury based on symptoms persisting ≥24 hours or until death, and other etiologies excluded C. Transient ischemic attack (TIA): a transient episode of neurological dysfunction caused by focal brain, spinal cord, or retinal ischemia without acute infarction Up to one-third of patients with symptoms lasting less than 24 hours are found to have an infarction. II. Epidemiology A. Stroke incidence: in the United States, ≈795,000 people experience new or recurrent strokes per year, with 130,000 deaths per year. B. Stroke prevalence: measures the total number of cases, new and old, at a particular time in a defined population; in the United States, stroke prevalence is 3,000,000. C. Stroke is the fifth most common cause of death in the United States, and leading cause of long-term disability. D. Between 1990 and 2010 the global stroke mortality has decreased. III. Frequency of Strokes TYPE Ischemic 87% Large-vessel disease 20% Cardioembolism 30% Small-vessel disease 20% Other 3% Unknown 27% Hemorrhage Subarachnoid hemorrhage (SAH) 3% Intracerebral hemorrhage (ICH) 10% IV. Pathophysiology A. Cerebral blood flow (CBF) Normal 50 cc/100 g/min Change in electrophysiologic activity 20 cc/100 g/min Irreversible ischemia 10 cc/100 g/min B. When blood supply is interrupted for 30 seconds, brain metabolism is altered: 1 minute, neuronal function ceases; 5 minutes, a chain of events that results in cerebral infarction ensues; evolution of an infarct: local vasodilatation → stasis of the blood column with segmentation of red cells; edema → necrosis of brain tissue. 1. Coagulation necrosis: the infarcted area is pale and swollen—blurred border between gray and white matter at 6 to 24 hours; “red neuron” (neuronal shrinkage and eosinophilia); astrocytes and oligodendrocytes; microglial cells disintegrate and give rise to somewhat granular appearance of the background; polymorphonucleocytes surround vessels; red cell extravasation; edematous swelling (may occur in 3–4 days). 2. Liquefaction (or absorption): represents removal of debris by macrophages 72 to 96 hours later; glitter cells—lipid-laden macrophages; sharpened demarcation between normal and infarcted tissue; tissue becomes mushy; there is hypertrophy (12–36 hours), then hyperplasia (48 hours to months) of astrocytes; macrophages clear debris at 1 cc/month. V. Risk Factors A. Nonmodifiable risk factors 1. Age: strongest determinant of stroke; incidence rises exponentially with age >65 years. 2. Sex: male 3. Race: in the United States, African American followed by Hispanic and Caucasian (extracranial > intracranial disease); Asian population has a higher incidence of intracranial stenosis. B. Modifiable risk factors 1. Hypertension: there is a fourfold increased risk of stroke with duration and severity of blood pressure (BP), especially among African Americans. It increases the risk by accelerating the progression of atherosclerosis and predisposing to small-vessel disease. Target blood pressure should be systolic BP <140/90. Choice of agent depends on comorbid conditions. 2. Cardiac disease: atrial fibrillation; valvular heart disease; myocardial infarction (MI), coronary artery disease (CAD), cardiomyopathy, a. Chronic nonvalvular atrial fibrillation stroke risk: 5% per year b. Valvular atrial fibrillation: 17-times-higher stroke risk c. Risk stratification is done using CHA2DS2 VASc score. CHA2DS2 VASC Congestive heart failure—1 point 1 point Hypertension—1 point 1 point Age 65–74 y—1 point 1 point ≥75 y—2 points 2 points Diabetes mellitus—1 point 1 point Stroke/TIA—2 points 2 points Vascular disease (e.g., peripheral artery disease, myocardial infarction, aortic plaque) 1 point Female sex 1 point Levels of risk for thromboembolic stroke Low risk = 0 points (antiplatelets) Moderate risk = 1 point (anticoagulation) High risk = ≥2 points (anticoagulation) d. Cardiomyopathy: stroke risk reduction for low-ejection fraction can be achieved with either anticoagulation or antiplatelets depending on the individual case. 3. Diabetes mellitus type 2: carries a 1.8 to 6.0 relative stroke risk a. Treatment: glycemic control with hemoglobin A1C <7 and hypertension control with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers 4. Blood lipids: relative stroke risk is 1.5. Treatment includes HMG-CoA reductase inhibitor with high doses for atherosclerotic disease. 5. Cigarette smoking: 1.9 increased risk for stroke; cessation reduces risk by 50% in 1 year and to baseline by 5 years. 6. Alcohol: a J-shaped relationship shows increased risk with moderate to heavy alcohol consumption (>14 oz of alcohol per month). 7. Asymptomatic carotid artery disease: >70% stenosis; risk is ~2% per year. Medical management with aspirin and HMG-CoA reductase inhibitor is recommended. Carotid endarterectomy (CEA) can be considered if perioperative risk is <3%. 8. Symptomatic carotid artery disease: >70% to 99% stenosis; risk of stroke is ~24.5% in 2 years. Greater benefit is obtained when CEA is performed within 2 weeks from stroke or TIA, if perioperative risk rate <6%. The 5-year absolute risk reduction with CEA for symptomatic carotid stenosis or >70% is 16. Benefit of CEA is lower in women, although patients who present with retinal ischemic symptoms have lower recurrent stroke risk as compared with hemispheric symptoms. Carotid artery stenting should be considered in patients who cannot undergo CEA. In patients with symptomatic carotid stenosis of 50% to 69%, the 5-year absolute risk reduction rate is 4.6%. 9. Symptomatic intracranial stenosis: if stroke or TIA (within 30 days) attributable to severe stenosis (70%–99%), the addition of clopidogrel 75 mg/d to aspirin 325 mg for 90 days along with maintenance of systolic BP below 140 mmHg and high-intensity HMG-Co-A reductase might be reasonable. Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) trial showed no benefit of anticoagulation compared with antiplatelets for symptomatic intracranial stenosis. Stenting vs. Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) and Vitesse Intracranial Stent Study for Ischemic Stroke Therapy (VISSIT) trial showed no benefit of intracranial stenting compared with aggressive medical management. 10. Most atherosclerosis occur in major cerebral arteries proximally at branching points: a. Bifurcation of common carotid artery into external carotid artery and internal carotid artery (ICA)—62% b. Origin of middle cerebral artery (MCA)—10% c. Origin of anterior cerebral artery (ACA)—1% d. Origin of vertebral artery—15% e. Others—11% C. Potential risk factors 1. Physical inactivity 2. Oral contraceptives: increased risk of cerebral venous thrombosis if concurrent hypercoagulable genetic condition; risk of ischemic stroke may be increased if patients have migraines. 3. Drug abuse a. Cocaine: risk of ischemic stroke may be up to 7 times higher; mechanism includes vasospasm and vasculitis. b. Opioids: septic emboli from intravenous drug abuse 4. Coagulopathy a. Lupus anticoagulant b. Heparin-induced thrombocytopenia 5. Others a. Heredity b. Aortic arch plaques c. Migraine d. Obstructive sleep apnea IV. Clinical Stroke Syndromes A. Common carotid: anatomy: the right common carotid artery arises from the brachiocephalic (innominate) artery, and the left common carotid directly from the aortic arch; the common carotids ascend to approximately the C4 level (just below the angle of the jaw) then divide into external and internal branches. 1. Internal carotid artery (ICA): anatomy: there are seven segments of the ICA: a. C1: cervical segment—branches: none b. C2: petrous segment—branches: vidian, caroticotympanic c. C3: lacerum segment—branches: none d. C4: cavernous segment—branches: meinigohypophyseal, inferolateral trunk, capsular e. C5: clinoid segment—branches: none f. C6: ophthalmic segment—branches: ophthalmic, superior hypophyseal g. C7: communicating segment—branches: posterior communicating, anterior choroidal i. Two mechanisms: embolus from an unstable plaque in ICA goes to distal vessel (artery-to-artery embolism) or occlusion (or severe stenosis) of carotid artery leads to hypoperfusion in the distal vasculature (also known as watershed or border-zone ischemia). ii. MCA/posterior cerebral artery (PCA) border zone: affects temporo-occipital portion of distal MCA territory; produces quadrant/hemianopic field defect, transcortical aphasia, or hemi-inattention (depending on hemisphere). iii. MCA/ACA border zone: affects superficial frontal and parietal parasagittal cortical area; produces proximal >> distal sensory motor deficit in the contralateral upper extremities; variable lower extremity involvement, sparing face and hand. iv. ICA nourishes optic nerve and retina: transient painless monocular blindness (amaurosis fugax) occurs in symptomatic carotid occlusion; stenosis, ulcerations, dissections of ICA may be a source of fibrin platelet emboli or may cause reduction of blood flow. 2. External carotid artery (ECA) branches and significance a. Superior thyroid artery b. Ascending pharyngeal artery—neuromeningeal branch supplies the dura and lower cranial nerves c. Lingual artery d. Facial artery—via the angular artery, anastomoses with branches of the ophthalmic artery e. Occipital artery—anastomoses with segmental branches of the vertebral artery f. Posterior temporal artery g. Superficial temporal artery Internal maxillary artery—a major branch, the middle meningeal artery enters the skull via the foramen spinosum; is a common cause of epidural hematoma. B. Middle cerebral artery (MCA): anatomy: four segments: M1—main MCA trunk with deep penetrating vessels and lenticulostriate arteries, M2—in the Sylvian fissure where the two divisions arise, M3—cortical branches, M4—over the cortical surface 1. Territory encompasses: a. Cortex and white matter of the inferior parts of frontal lobe, including areas four and six, centers for lateral gaze, Broca’s area b. Cortex and white matter of parietal lobe, including sensory cortex and angular and supramarginal c. Superior parts of the temporal lobe and insula, including Wernicke’s area d. Penetrating branches: putamen, outer globus pallidus, posterior limb of internal capsule, body of caudate, corona radiata 2. Stem occlusion: blocking deep penetrating and superficial cortical branches—contralateral hemiplegia (face, arm, and leg), hemianesthesia, homonymous hemianopia, deviation of head and eyes toward side of the lesion; left hemisphere lesions—global aphasia; right hemisphere—anosognosia and amorphosynthesis 3. Superior division: supplies rolandic and prerolandic areas—dense sensorimotor of face and arm >> leg; ipsilateral deviation of head and eye; brachiofacial paralysis, no impairment of consciousness; left-sided lesions—initial global aphasia, then predominantly motor a. Ascending frontal branch: initial mutism and mild comprehension defect, then slightly dysfluent, agrammatic speech with normal comprehension b. Rolandic branches: sensorimotor paresis with severe dysarthria but little aphasia c. Cortical-subcortical branch: brachial monoplegia d. Ascending parietal: no sensorimotor defect, only a conduction aphasia 4. Inferior division: left sided—Wernicke’s aphasia; right sided—left visual neglect; superior quadrantanopia or homonymous hemianopia; agitated confusional state from temporal lobe damage 5. Other cortical syndromes a. Dominant parietal lobe: Gerstmann syndrome—finger agnosia, acalculia, right-left confusion, alexia (supramarginal gyrus), alexia with agraphia (angular gyrus), ideational apraxia b. Nondominant parietal lobe: anosognosia, autoprosopagnosia, neglect, constructional apraxia, dressing apraxia c. Bilateral anterior poles of the temporal lobes: Klüver-Bucy syndrome; docility, hyper-oral, hypersexual, hypomobile, hypermetamorphosis, visual agnosia In addition to prosopagnosia (deficit in facial recognition), other nondominant hemisphere deficits include auditory agnosia (deficit in recognition of sounds), autotopagnosia (inability to localize stimuli on the affected side), and phonagnosia (inability to recognize familiar voices). Pure word deafness (inability to recognize spoken language) is a dominant hemisphere deficit! d. Aphasias C. Anterior cerebral artery (ACA): supplies anterior three-fourths of the medial surface of cerebral hemisphere, including medial-orbital surface of frontal lobe, strip of lateral surface of cerebrum along the superior border, anterior four-fifths of corpus callosum, deep branches supplying anterior limb of internal capsule, inferior part of caudate, anterior globus pallidus 1. Stem occlusion: proximal to the anterior communicating artery, usually well tolerated; if both arteries arise from one ACA, paraplegia, abulia, motor aphasia, frontal lobe personality changes; distal to the anterior communicating artery—sensorimotor defect of contralateral foot >> shoulder and arm; motor in foot and leg >> thigh; sensory is more of discriminative modalities and is mild or absent; head and eyes deviated ipsilaterally, urinary incontinence, contralateral grasp reflex, paratonic rigidity (gegenhalten); left sided—may have alien hand. 2. Branch occlusions: fragments of the total syndrome (usually spastic weakness and cortical sensory loss of foot or leg); occlusion of Heubner’s artery: may give rise to transcorticomotor aphasia 3. Penetrating branches: transient hemiparesis, dysarthria, abulia or agitation; left side—stuttering and language difficulty; right side—visuospatial neglect; bilateral caudate—syndrome of inattentiveness, abulia, forgetfulness, sometimes agitation and psychosis D. Anterior choroidal artery: long narrow artery from ICA just above the posterior communicating artery; supplies internal globus pallidus, posterior limb of internal capsule, contiguous structures such as the optic tract; choroid plexus of lateral ventricles; clinical: contralateral hemiplegia, hemihypesthesia, homonymous hemianopsia; cognitive function is spared; no uniform syndrome E. Posterior cerebral artery (PCA): in 70%, both PCAs originate from the bifurcation of the basilar artery; in 20% to 25%, one of the PCAs comes from the ICA in the remainder, both PCAs from ICA. (Fetal origin of the PCA: posterior communicating artery diameter is large and the ipsilateral P1 segment is hypoplastic.) 1. Anatomy a. Interpeduncular branches/mesencephalic artery: supply red nucleus, substantia nigra, medial cerebral peduncles, medial longitudinal fasciculi, medial lemnisci b. Thalamoperforate/paramedian thalamic arteries: inferior, medial, and anterior thalami c. Thalamogeniculate branches: geniculate body, posterior thalamus d. Medial branches: lateral cerebral peduncles, lateral tegmentum, corpora quadrigemina, pineal gland e. Posterior choroidal: posterosuperior thalamus, choroid plexus, posterior hypothalamus, psalterium (decussation of fornices) f. Cortical branches: inferomedial temporal lobe, medial occipital, including lingula, cuneus, precuneus, and visual areas 17, 18, and 19 2. Syndromes a. Anterior and proximal syndromes, involves interpeduncular, thalamic perforant, thalamogeniculate branches i. Thalamic syndrome of Dejerine and Roussy: infarction of sensory relay nuclei (due to occlusion of thalamogeniculate)—deep and cutaneous sensory loss contralateral, with transient hemiparesis; after an interval, pain, paresthesia, hyperpathia of affected parts; distortion of taste, athetotic posturing of hand; depression ii. Central midbrain and subthalamic syndromes: due to occlusion of interpeduncular branches; oculomotor palsy with contralateral hemiplegia (Weber syndrome), palsies of vertical gaze, stupor, coma, movement disorders (usually contralateral ataxic tremor) iii. Anteromedial-inferior thalamic syndromes: occlusion of thalamoperforate branches; hemiballismus, hemichoreoathetosis; deep sensory loss, hemiataxia, tremor; occlusion of dominant dorsomedial nucleus gives rise to Korsakoff syndrome b. Cortical syndromes i. Occlusion of branches to temporal and occipital lobes: homonymous hemianopsia; macular and central vision may be spared owing to collateralization of occipital pole from distal branches of MCA (or ACA); visual hallucination in blind parts (Cogan) or metamorphopsia, palinopsia ii. Dominant hemisphere: alexia, anomia (most severe for colors and visually presented material—may describe their function and use them but not name them), visual agnosia, occasional memory impairment c. Bilateral cortical syndromes: result of successive infarctions from embolus or thrombus of upper basilar artery i. Cortical blindness: blindness with unformed visual hallucinations; pupillary reflexes preserved, optic discs normal; patient may be unaware (Anton syndrome) ii. If confined to occipital poles, may have homonymous central scotomas iii. Balint’s syndrome: from bilateral occipital-parietal border zones (A) Oculomotor apraxia (B) Optic ataxia (failure to grasp objects under visual guidance) (C) Asimultanagnosia iv. Bilateral inferomedial temporal lobes: Korsakoff amnestic state v. Bilateral mesial-temporal-occipital lesions: prosopagnosia F. Vertebral artery 1. Anatomy: chief arteries of the medulla; supplies lower three-fourths of pyramid, medial lemniscus, all of lateral medullary region, restiform body, posterior-inferior part of cerebellar hemisphere; long extracranial course and passes through transverse processes of C6 through C2 before entering the cranial cavity—may be subject to trauma, spondylotic compression. 2. Syndromes a. Lateral medullary syndrome/Wallenberg syndrome: vestibular nuclei (nystagmus, oscillopsia, vertigo, nausea, vomiting); spinothalamic tract (contralateral impairment of pain and thermal sense over one-half the body); descending sympathetic tract (ipsilateral Horner’s—ptosis, miosis, anhidrosis); cranial nerves (CNs) IX and X (hoarseness, dysphagia, ipsilateral paralysis of palate and vocal cord, diminished gag); otolithic nucleus (vertical diplopia and illusion of tilting of vision); olivocerebellar and/or spinocerebellar fibers/restiform body (ipsilateral ataxia of limbs, falling to ipsilateral side); nucleus and tractus solitarius (loss of taste); descending tract and nucleus of V (pain, burning, impaired sensation on ipsilateral one-half of face; rarely nucleus cuneatus and gracilis (ipsilateral numbness of limbs); most likely due to occlusion of vertebral artery or posterior-inferior cerebellar artery. b. Opalski syndrome: considered a variant of lateral medullary syndrome with ipsilateral hemiplegia, likely due to caudal extension of the infarct due to involvement of perforator branches arising from the distal vertebral artery. c. Medial medullary syndrome: involves medullary pyramid (contralateral paralysis of arm and leg); medial lemniscus (contralateral impaired tactile and proprioceptive sense over one-half the body); CN XII (ipsilateral paralysis and, later, hemiatrophy of the tongue). d. Hemimedullary infarction (Babinski–Nageotte syndrome): occlusion of the ipsilateral vertebral artery proximal to the posterior-inferior cerebellar artery and its anterior spinal artery causes medial medullary syndrome and lateral medullary syndrome simultaneously. e. Posterior medullary region: ipsilateral cerebellar ataxia and, rarely, hiccups. f. Avellis syndrome: tegmentum of medulla: CN X, spinothalamic tract (paralysis of soft palate and vocal cord and contralateral hemianesthesia). g. Jackson syndrome: tegmentum of medulla: CN X, XII, corticospinal tract (Avellis syndrome plus ipsilateral tongue paralysis). G. Basilar artery 1. Branches a. Paramedian b. Short circumferential (supplying lateral two-thirds of pons and middle and superior cerebellar peduncles) c. Long circumferential (anterior-inferior cerebellar artery and superior cerebellar artery) d. Paramedian (interpeduncular) at the bifurcation of the basilar artery supplying subthalamic and high midbrain 2. Syndromes a. Basilar artery syndrome: bilateral corticobulbar and corticospinal tracts (paralysis/weakness of all extremities plus all bulbar musculature); ocular nerves, medial longitudinal fasciculus, vestibular apparatus (diplopia, paralysis of conjugate gaze, internuclear ophthalmoplegia, horizontal and/or vertical nystagmus); visual cortex (blindness; visual field defects); cerebellar peduncles and hemispheres (bilateral cerebellar ataxia); tegmentum of midbrain/thalami (coma); medial lemniscus-spinothalamic tracts (may be strikingly intact, syringomyelic, reverse, or involve all modalities) b. Medial inferior pontine syndrome (occlusion of paramedian branch of basilar artery): paramedian pontine reticular formation (paralysis of conjugate gaze to the side of lesion but preservation of convergence); vestibular nuclei (nystagmus); middle cerebral peduncle (ipsilateral ataxia of limbs and gait); CN VI (ipsilateral diplopia on lateral gaze), corticobulbar and corticospinal tract (contralateral paresis of face, arm, and leg); medial lemniscus (contralateral tactile dysfunction and proprioceptive sense over one-half the body) c. Lateral inferior pontine syndrome (occlusion of anterior-inferior cerebellar artery): CN VIII (horizontal and vertical nystagmus, vertigo, nausea, oscillopsia, deafness, and tinnitus); CN VII (ipsilateral facial paralysis); paramedian pontine reticular formation (paralysis of conjugate gaze to side of lesion); middle cerebellar peduncles and cerebellar hemisphere (ipsilateral ataxia); main sensory nucleus and descending tract of V (ipsilateral impairment of sensation over face); spinothalamic tract (contralateral impairment of pain and thermal sense over one-half the body) d. Millard-Gubler syndrome (base of pons): CN VI and VII and corticospinal tract (facial and abducens palsy plus contralateral hemiplegia) e. Medial midpontine syndrome (paramedian branch of midbasilar artery): middle cerebellar peduncle (ipsilateral ataxia of limbs and gait); corticobulbar and corticospinal tracts (contralateral paralysis of face, arm, and leg; deviation of eyes); medial lemniscus (variable—usually pure motor) f. Lateral midpontine syndrome (short circumferential artery): middle cerebellar peduncle (ipsilateral ataxia); motor nucleus of V (ipsilateral paralysis of masticatory muscles); sensory nucleus of V (ipsilateral sensory facial impairment) g. Medial superior pontine syndrome (paramedian branches of upper basilar artery): superior and middle cerebellar peduncle (ipsilateral cerebellar ataxia); medial longitudinal fasciculus (ipsilateral internuclear ophthalmoplegia); central tegmental bundle (rhythmic myoclonus of palate, pharynx, vocal cords, etc.); corticobulbar and corticospinal tracts (contralateral paralysis of face, arm, and leg); medial lemniscus (rarely with sensory impairment) h. Lateral superior pontine syndrome (syndrome of superior cerebellar artery): middle and superior cerebellar peduncles, dentate nucleus (ipsilateral ataxia, falling to side of lesion); vestibular nuclei (dizziness, nausea, horizontal nystagmus); descending sympathetic fibers (ipsilateral Horner’s); spinothalamic tract (contralateral impairment of pain and temperature sense of face, limb, trunk); medial lemniscus—lateral portion (contralateral impaired touch, vibration, position sense of leg >> arm); other—ipsilateral paresis of conjugate gaze, skew deviation i. Base of midbrain (Weber syndrome): CN III (ipsilateral oculomotor palsy) plus corticospinal tract (crossed hemiplegia) j. Tegmentum of midbrain (Claude syndrome): CN III, red nucleus, and brachium conjunctivum (contralateral cerebellar ataxia and tremor) k. Benedikt syndrome: CN III, red nucleus, plus corticospinal tract l. Nothnagel syndrome: CN III (unilateral or bilateral); superior cerebellar peduncles (ocular palsies, paralysis of gaze, cerebellar ataxia); usually caused by a tumor m. Parinaud syndrome: dorsal midbrain—supranuclear mechanism for upward gaze and other structures in periaqueductal gray (paralysis of upward gaze and accommodation, fixed pupils) VII. TIA A. Etiology: causes for TIA are the same as those for ischemic stroke. Management of TIA mainly includes risk stratification and identifying a treatable cause. B. Presentation and subsequent stroke risk: presenting symptoms are often similar to ischemic stroke symptoms but usually last for a few minutes. Estimated risk of stroke 2 days after TIA is around 4%, and in the next 90 days is around 9%. The Age, Blood pressure, Clinical presentation, Duration of symptoms, Diabetes (ABCD2) score can be used to stratify the stroke risk after a TIA. (See accompanying table.) C. Management: monitor patients for 24 hours for symptom recurrence. Brain MRI to evaluate for acute ischemic stroke or for old stroke burden, arterial imaging, and cardiac evaluation including rhythm monitoring and echocardiogram. Patients should be started on aspirin unless there is indication for anticoagulation. Clopidogrel in High-risk patients with Acute Nondisabling Cerebrovascular Events (CHANCE) study showed benefit of dual antiplatelet therapy (aspirin + clopidogrel) for 21 days, after a TIA or mild ischemic stroke. VIII. Lacunar Stroke: These are usually <15 mm in diameter and occur due to occlusions of penetrating small-end arteries of the anterior or posterior circulation. A. Pathology: lipohylinosis, or atherosclerosis, underlies small-vessel occlusion related to hypertension primarily, but diabetes and hyperlipidemia might play a role. B. Syndromes and locations 1. Pure motor hemiparesis: internal capsule, adjacent corona radiata, paramedian pons, or medullary pyramid 2. Pure sensory stroke: ventral posterior thalamus 3. Sensory-motor stroke: thalamus, corona radiata 4. Clumsy-hand dysarthria: anterior limb of the internal capsule, genu, or pons 5. Ataxia hemiparesis: pons, internal, capsule, deep grey nuclei, cerebellum IX. Acute Stroke Therapy A. Supportive care and treatment: all stroke patients regardless of the type must be first stabilized with regard to airway, breathing, and circulation (ABCs). Once the patient is stabilized, efforts should be made to identify the exact time the patient was last seen well. Keep in mind that symptom onset may be reported when the patient was found, which is different from when patient was last seen normal. Evaluation should also rule out any potential stroke mimics. The National Institute of Health Stroke Scale (NIHSS) performed at the bedside will help establish the severity of the stroke. A noncontrast CT scan of the head must be performed to rule out hemorrhagic stroke. B. Thrombolysis therapy: Once a hemorrhagic stroke is ruled out with a CT scan, all efforts should be made to establish reperfusion for ischemic stroke. 1. Intravenous: The National Institute of Neurological Disorders and Stroke (NINDS) Tissue Plasminogen Activator (tPA) trial established the efficacy of intravenous (IV) tPA for patients presenting within 3 hours from symptom onset. Compared with the placebo group, patients treated with tPA had a 16% absolute increase in favorable outcomes at 3 months. IV tPA is approved by the U.S. Food and Drug Administration (FDA) for ischemic stroke presenting within 3 hours from last known normal. No consent is required for this time window. See the accompanying table for inclusion/exclusion criteria and testing recommended prior to initiation of IV tPA. For patients presenting after 3 hours but before 4.5 hours, the European Cooperative Acute Stroke Study (ECASS) III was the first study to show a statistical benefit for IV tPA use with a slightly higher intracranial hemorrhage rate. The American Heart Association and American Stroke Association recommend the use of IV tPA for patients (see inclusion/exclusion criteria in the following table) presenting between 3 and 4.5 hours from last known normal; however, this is not FDA approved. 2. Orolingual angioedema that can occur due to IV tPA varies from a rate of 1.3% to 5.1% and is usually mild, transient, and contralateral to the ischemic hemisphere. Increased risk of angioedema is associated with ACE inhibitor use. Treatment includes close monitoring, intravenous ranitidine, diphenhydramine, and methylprednisolone.
 NB:
NB:
 NB:
NB:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






