13 | Subaxial Subluxation of the Rheumatoid Cervical Spine |
 | Case Presentation |
History and Physical Examination
A 54-year-old female patient with a past medical history significant for rheumatoid arthritis (RA) for which she had been treated with disease-modifying, antirheumatic drugs, including prednisone, for the past 17 years was referred for evaluation of neck pain and occipital headaches. These symptoms had gradually worsened over the past 6 months.
She stated that she had had difficulty with fine motor skills of her hands for many years secondary to hand deformities, but over the past few months had felt more “clumsy.” She was dropping objects more frequently. She was also more unsteady on her feet, having fallen to the ground several times over the past 2 months. She denied any changes in urinary or bowel control.
Physical examination revealed a painful, decreased cervical range of motion. She noted a clunking sensation when her neck was in extension (positive Sharp-Purser test). Neurological examination was notable for a Lhermitte sign with symptoms down her right arm and torso. Motor strength was difficult to assess secondary to pain and peripheral changes. Sensory and cranial nerve exams were grossly intact. She had 3+ reflexes throughout the upper extremities (including an inverted radial component on the right), 2+ reflexes in the lower extremities, and bilaterally positive Hoffmann and Babinski signs, five to six beats of clonus, and a wide-based gait (Fig. 13–1).
Radiological Findings
Anteroposterior and lateral radiographs (Fig. 13–2) demonstrate extensive degenerative changes, atlantoaxial subluxation (AAS), as well as multilevel subaxial subluxations (SASs) (C2-3, C4-5, and C7-T1 of 3.9 mm, 3.6 mm, and 3.2 mm, respectively, with space available for the cord (SAC) of 10.8 mm, 9.5 mm, and 10.1 mm, respectively). Fig. 13–3 is the lateral flexion and extension radiographs, which show reduction of C1-2 and C2-3 with extension and static anterolisthesis of C4-5 and C7-T1. Fig. 13–4 shows representative sagittal computed tomographic (CT) and magnetic resonance (MR) images.
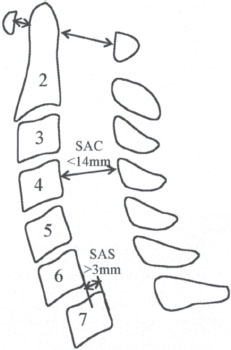
Figure 13–1 Illustration showing the cervical measurements of sub-axial subluxations (SASs) and space available for the cord (SAC). (From Grauer JN, Tingstad EM, Rand N, Christie MJ, Hilibrand AS. Predictors of paralysis in the rheumatoid cervical spine in patients undergoing total joint arthroplasty. J Bone Joint Surg Am 2004;86:1420-1424. Adapted with permission.)
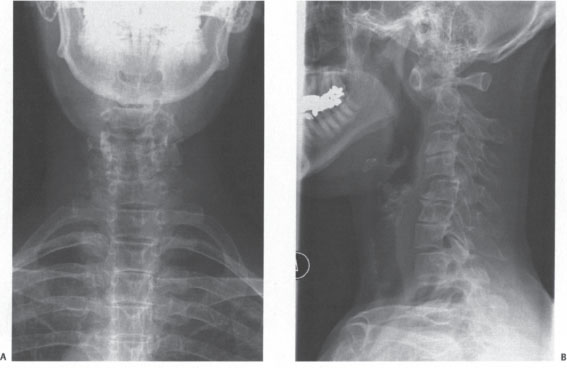
Figure 13–2 Anteroposterior and lateral radiographs of the case example showing degenerative changes and C1-2, C2-3, C4-5, C7-T1 anterolistheses.
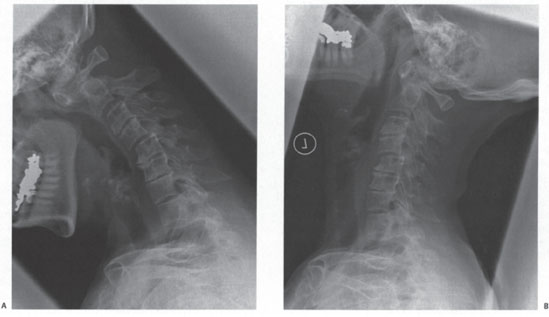
Figure 13–3 (A) Lateral flexion and (B) extension radiographs demonstrate reduction of C1 -2 and C2-3 with extension and static anterolisthesis of C4-5 and C7-T1.
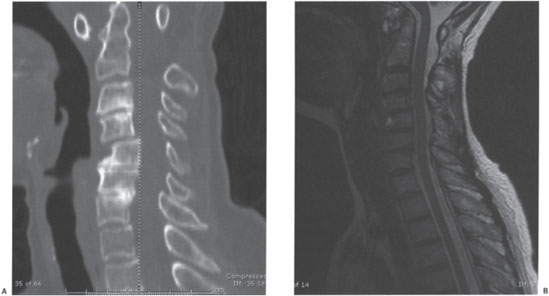
Figure 13–4 (A) Sagittal computed tomographic and (B) magnetic resonance images of the case example highlighting bony and neural element anatomy.
Diagnosis
Based on history, physical examination, and dynamic radiographs, a diagnosis of subaxial instability and myelopathy was made.
 | Background |
RA is a systemic autoimmune disease of unknown etiology that primarily involves the synovium. The local milieu created by the inflammatory process leads to destruction of the articular joint surfaces, joint capsules, and adjacent ligaments.1 Although the joints of the hands and feet are most commonly affected,2 cervical spine involvement is also common due to the important stabilizing role of synovial articulations, such as those at C1-2 and the facet joints throughout the subaxial spine.3 In fact, Winfield et al showed that most patients have radiographic changes of the cervical spine within the first 2 years of diagnosis of RA.4
The erosive changes in the cervical spine associated with RA may lead to instability, deformity, and neurological sequelae.1 These changes fall into three categories: AAS, basilar invagination (BI), and SAS. AAS is the most common, representing 66% of rheumatoid cervical subluxations. BI is seen in 20% and is almost always preceded by AAS. Finally, SAS is seen in 20 to 25% of patients with cervical subluxations.5–7 The previous chapter addresses AAS and BI, whereas this chapter concentrates on SAS.
SAS is a result of erosions of the facet and diskovertebral joints8 and represents a relatively late manifestation of rheumatoid cervical spine disease.2,7,9 This occurs most frequently at the C2-3 and C3-4 levels, yet often affects multiple adjacent levels yielding in a characteristic “staircase” deformity.10,11 There are several different definitions of subaxial subluxations.
In evaluating the natural history of cervical changes with RA, Fujiwara et al followed 161 patients with nonoperative treatment for a minimum of 5 years (range 5 to 20 years).12 At the beginning of the study, four patients had SAS. During the follow-up period, 14 patients developed SAS. Seventeen of the 18 patients with SAS at the end of the study had upper cervical changes as well. They reported that the mean duration of the RA at the onset of SAS was 13.4 years. Generally, SAS progressed from a reducible lesion to an irreducible lesion when dynamic radiographs were evaluated. Other authors have similarly observed that the major risk factor for the development of cervical subluxation is the severity and duration of the underlying rheumatoid disease2,9 and steroid use.13
Yamauchi et al postulated that as the motion of the upper cervical spine decreases, increasing stress is transmitted to the lower cervical spine, leading to subaxial instability.14 Kraus et al demonstrated that occipital-cervical fusion correlated with a significantly higher incidence of postsurgical SAS than did atlantoaxial fusion (36% vs 5.5%).16 Matsunaga et al demonstrated an association between the position of the occiput and the axis (O–C2 angle) with occipital-cervical fusion postoperative SASs. The O–C2 angle was defined as the angle between the McGregor line and the inferior surface of the axis. Angles clockwise relative to the inferior surface of the axis were indicated as plus (+) and those counterclockwise as minus (−). Therefore, plus (+) refers to angles where the head was fixed/fused in extension and minus (−) in flexion. The rate of occurrence of SAS was significantly higher in patients with an abnormal minus O–C2 angle (85.7%) than in patients with a normal (0 to 30 degrees) or a plus O–C2 angle (4.1 %).17 Given the association of upper cervical fusion with SASs, it would be optimal to have criteria to be able to predict which patients have a higher probability to develop middle and lower cervical spine changes after surgical fusion of the upper cervical spine. This information could then be used to determine the optimal extent of the cervical fusion. Matsunaga et al used a computer-assisted biomechanical analysis of preoperative and postoperative lateral dynamic radiographs to determine the two-dimensional buckling alignment of the cervical spine of 25 rheumatoid patients and 15 nonrheumatoid patients with atlantoaxial dislocation who underwent occipitocervical fusion.18 They were able to demonstrate that a preoperative cervical buckling over 10×104 constitutes a predictive risk factor for SAS after O–C fusion.18 Thus a biomechanical analysis on the basis of the presence or absence of buckling preoperatively may prove useful in predicting postoperative subluxation, and therefore, may indicate a more extensive spinal fusion or prevent unnecessary prophylactic surgery.
A careful cervical history and physical examination are certainly crucial in the evaluation of the RA population. The most common presenting complaint of a rheumatoid patient with cervical instability is pain.2,7 This may be occipital, axial, or radicular and may be exacerbated by neck motion. Additional myelopathic complaints may be present. Examination is paramount but may be limited due to peripheral RA manifestations such as hand changes.19 In comparison to patients with AAS, patients with SAS have a higher incidence of neurological deficit.20
Indications for surgical intervention have been suggested. Most authors agree that those with myelopathy, progressive neurological deficit, or instability with intractable pain warrant surgical intervention.1,6,16,21–24 However, controversy exists regarding surgery for asymptomatic patients with radiographic abnormalities (Ranawat class I) or nonambulatory (Ranawat class IIIB) patients. Ranawat et al classified rheumatoid patients based on neural deficits: class I meant no neural deficit; class II meant subjective weakness with hyperreflexia and dysesthesia; class IIIA meant ambulatory patients with objective weakness and long-tract signs; and class IIIB meant nonambulatory quadriparetic patients.23 Although the latter patients are obviously myelopathic, some reported surgical results have been poor with high mortality rates.20,25 Therefore, one must consider the patients’ comorbidities and surgical risks when considering possible surgical intervention.
Boden et al evaluated a large cohort of patients (n = 73) who were followed over a 20-year period for instability associated with RA. Although only patients with neurological deficit underwent surgery in their series, they recommended surgery for patients with or without a neurological deficit if certain radiographic criteria were met. They found that the diameter of the subaxial canal, or SAC, was found to correlate more closely with paralysis than with the degree of displacement of one vertebral body on another. Of the 10 patients with SAS, eight had a Ranawat class III deficit and all eight had a SAC of 13 mm or less. The other two patients were Ranawat class II and both had a SAC of 14 mm. Thus a SAC, or posterior atlantodens interval (PADI), of < 14 mm was predictive of paralysis and should lead to surgical considerations. This study also showed that surgical intervention before the onset of significant paralysis resulted in better neurological outcomes.21
Knowing the high incidence of radiographic instability, even in asymptomatic rheumatoid patients, Sharp and Purser recommend lateral flexion and extension radiographs in all patients with severe RA who will be undergoing general anesthesia.26 These screening radiographs include dynamic flexion-extension views because SAS may not be apparent on the neutral lateral.
To further assess the importance of preoperative cervical flexion and extension radiographs in rheumatoid patients Collins et al retrospectively analyzed 113 patients who had undergone a total hip or knee arthroplasty.27 Sixty-nine (61%) of the patients had radiographic instability (24, or 21%, with SAS), of which 35 (50%) had no signs or symptoms of instability. Grauer et al retrospectively found that four of 65 (8%) patients who underwent a total hip or knee arthroplasty had radiographic signs that were predictive of paralysis, as defined by Boden (PADI or SAC < 14 mm)21 compared with the 20 (41%) patients with traditional radiographic evidence of instability (AADI > 3 mm or SAS > 3 mm).28 Although there is some variability of the reported incidence of cervical instability in this population, these studies do emphasize the importance of preoperative screening films in rheumatoid patients who are to undergo endotracheal intubation.
CT can be helpful in defining the bony anatomy of the subaxial spine and can be particularly helpful at defining the anatomy of the occipital-cervical junction. Magnetic resonance imaging (MRI) is important to define compression of the neural elements and soft tissue involvement, such as pannus.20 Increased signal intensity within the spinal cord on T2-weighted images, which may represent edema, gliosis, or myelomalacia, has been associated with poor neurological recovery following surgery.29 Nonetheless, MRI may underestimate neural element compression due to the extended posture of the neck with a standard supine MRI. Bell and Stearns recommend dynamic MRI to determine the most caudal level needed to decompress/fuse.30 Although being used less commonly with the advent of MRI, CT myelograms are also useful in assessing neural compression if an MRI is contraindicated.31
 | Authors’ Preferred Method of Surgical Management |
The previous chapter covered surgical considerations for upper cervical instabilities. Certainly any such interventions must take potential SASs into account and probably incorporate those into the fusion construct. In fact, multiple studies have shown previously undiagnosed SASs after upper cervical fusion operations.1,16,24,32 Whether these subaxial lesions represent the natural history of the patients’ RA or are secondary to higher forces generated by a longer lever arm can only be postulated. As such, patients who have undergone upper cervical fusions must be followed closely for development of SASs.
SAS unto itself may also lead to the recommendation for surgical intervention. Symptomatic SAS, or those with a SAC ≤ 14 mm, are best considered for surgical intervention. This is most commonly approached posteriorly with decompression and fusion.24 If there is reduction of the deformity with extension, and neural element compression is alleviated in this posture, fusion alone may be considered. Alternatively, anterior decompression and fusion may be considered if there is a focal, reducible subluxation. Anterior procedures may also be combined with posterior decompression and fusion if longer constructs are considered for a patient who cannot achieve a neutral or extended cervical posture with preoperative extension.
In the case example, the patient had a combination of reducible and fixed SAS as well as reducible AAS. Because the patient’s lateral extension film demonstrated an overall lordotic picture, we recommend a posterior decompression and fusion. Given that there was involvement from upper cervical to T1, a C1 to the upper thoracic procedure would generally be recommended. Proximal fixation could be achieved with C1 lateral mass screws, Magerl screws, or extension to the occiput. Midcervical fixation is generally achieved with lateral mass fixation. Lower cervical and upper thoracic fixation can be achieved with pedicle screw fixation.33,34 Alternatively, one could consider a procedure to C5 with close monitoring of the cervicothoracic junction; however, the patient would have to be counseled as to the potential need for extension of the surgical construct.
 | Postoperative Care |
All patients are immobilized for ~6 to 12 weeks status postfusion for RA-related instability. This is most often a hard cervical collar. However, if there is concern about fixation due to the construct or poor bone quality, a halo may be considered. If fusion is extended to the thoracic spine, a cervicothoracic orthosis may be considered.
Most spine surgeons follow patients with intermittent anteroposterior and lateral radiographs intermittently during the postoperative braced period. Once a patient has been weaned from the orthosis, flexion-extension radiographs are appropriately considered. Despite solid fusion, patients must be followed closely for development of delayed instability above or below the surgical construct.
 | Complications |
The overall complication rate in patients with RA is significantly higher than that of the general population.13 This may be due to the underlying condition or to the medications used for its treatment. Chronic steroid use and subsequent osteopenia may lead to increased rates of instrumentation failure: implant cutout, graft settling, graft resorption, and progressive vertebral collapse.23 Pseudarthrosis is also of significant concern.7,23,35
Rheumatoid patients may be a greater risk for other complications, such as infection, if they are on long-term corticosteroids or other immunosuppressants. Close attention should be paid to their skin integrity, especially in the bedridden, to prevent decubitus ulcers at prominences such as the occiput.23,36 This population is also at increased risk for aspiration pneumonia. Ranawat class III patients, especially class IIIB, have an extremely high mortality rate.23,25,35
As previously discussed, recurrent instability in the form of SAS, either from disease progression or from increased force at the cervical disk caudal to a prior fusion, may occur.14–18 This can result in either or both instability and potential neurological injury and may require revision surgery with extension of the fusion to include the involved levels.
 | Alternate Methods of Management |
Nonsurgical Management
Certainly, the mainstay of treatment for most spinal conditions is conservative, if possible. Although the factors described earlier may lead to the clear recommendation for surgical intervention; this must be considered in light of a patient’s comorbid medical conditions. End-stage rheumatoid patients may present with factors prohibiting surgical intervention.
Thus traction or external immobilization or both may be warranted. Oostveen et al reported two cases of rapidly progressing cervical myelopathy who obtained improvement of myelopathic symptoms and stabilization of their subaxial cervical lesions with prolonged traction and halo immobilization alone.37 They suggested cervical traction and immobilization with a halo vest could be considered an independent treatment option for high-risk surgical candidates.
Laminoplasty
Laminoplasty may be considered when there is no preoperative cervical kyphotic deformity and mild SAS (≤ 5 mm) and when the main symptom is myelopathy and not neck pain.38,39 Mukai et al retrospectively reviewed a cohort of SAS cases treated with laminoplasty. They found improvement in myelopathy for patients with nonmutilating-type RA (less than 40 eroding joints) and SAS ≤ 5 mm.38 Of course, there is concern for worsening of underlying subluxation if such an intervention is performed.
 | Conclusion |
SASs tend to occur in later and in more severe forms of RA. Close monitoring of rheumatoid patients with evidence of cervical instability should lead to earlier surgical stabilization and, therefore, better outcomes. Multiple studies have shown that long-term mortality increases, perioperative complications decrease, and neurological recovery decreases once a patient becomes myelopathic. Boden et al showed that a preoperative SAC < 14 mm was associated with more severe preoperative paralysis (Ranawat class III deficit) and that a preoperative SAC ≥ 14 mm was a positive predictor for full neurological recovery.21 Therefore, Boden et al supplied us with a radiographic value from which we can make surgical decisions. Close monitoring and appropriate surgical intervention are appropriate for this population.
References
1. Simpson JM, An HS, Balderston RA. Complications of surgery of the spine in rheumatoid arthritis and ankylosing spondylitis. In: Balderston RA, An HS, eds. Complications in Spinal Surgery. Philadelphia: WB Saunders; 1991:169–175
2. Bland JH. Rheumatoid arthritis of the cervical spine. J Rheumatol 1974;3:319–342
3. Hughes JT. Spinal cord involvement by C4–C5 vertebral subluxation in rheumatoid arthritis: a description of 2 cases examined at necropsy. Ann Neurol 1977;1:575–582
4. Winfield J, Cooke D, Brook AS, Corbett M. A prospective study of the radiological changes in the cervical spine in early rheumatoid arthritis. Ann Rheum Dis 1981;40:109–114
5. Dreyer SJ, Boden S. Natural history of rheumatoid arthritis of the cervical spine. Clin Orthop Relat Res 1999;366:98–106
6. Lipson SJ. Rheumatoid arthritis in the cervical spine. Clin Orthop Relat Res 1989;239:121–127
7. Rawlins BA, Girardi FP, Boachie-Adjei O. Rheumatoid arthritis of the cervical spine. Rheum Dis Clin North Am 1998;24:55–65
8. Martel W. Pathogenesis of cervical discovertebral destruction in rheumatoid arthritis. Arthritis Rheum 1977;20:1217–1225
9. Smith PH, Benn RT, Sharp J. Natural history of rheumatoid cervical luxations. Ann Rheum Dis 1972;31:431–439
10. Paimela L, Laasonen L, Kankaanpaa E, Leirsisalo-Repo M. Progression of cervical spine changes in patients with early rheumatoid arthritis. J Rheumatol 1997;24:1280–1284
11. Yonezawa T, Tsuji H, Matsui H, Hirano N. Subaxial lesions in rheumatoid arthritis: radiographic factors suggestive of lower cervical myelopathy. Spine 1995;20:208–215
12. Fujiwara K, Owaki H, Fujimoto M, Yonenobu K, Ochi TA. Long-term follow-up cervical lesions in rheumatoid arthritis. J Spinal Disord 2000;13:519–526
13. Pisitkun P, Pattarowas C, Siriwongpairat P, Totemchokchyarkarn K, Nantiruj K, Janwityanujit S. Reappraisal of cervical spine subluxation in Thai patients with rheumatoid arthritis. Clin Rheumatol 2004;23:14–18
14. Yamauchi K, Nekozuka Y, Kasai Y, et al. Natural history of rheumatoid cervical spine. Jpn J Rheum Joint Surg 1987;6:481–489
15. Yoshida K, Hanyu T, Takahashi H. Progression of rheumatoid arthritis of the cervical spine: radiographic and clinical evaluation. J Orthop Sci 1999;4:399–406
16. Kraus DR, Peppelman WC, Agarwal AK, et al. Incidence of subaxial subluxation in patients with generalized rheumatoid arthritis who have had previous occipital cervical fusions. Spine 1991;16: S486-S489
17. Matsunaga S, Onishi T, Sakou T. Significance of occipitoaxial angle in subaxial lesion after occipitocervical fusion. Spine 2001; 26:161–165
18. Matsunaga S, Sakou T, Sunahara N, Oonishi T, Maedo S, Nakanisi K. Biomechanical analysis of buckling alignment of the cervical spine: predictive value for subaxial subluxation after occipitocervical fusion. Spine 1997;22:765–771
19. Bailey RW. Rheumatoid arthritis and other noninfectious inflammatory diseases. In: The Cervical Spine Research Society. The Cervical Spine. Philadelphia: Lippincott-Raven; 1983
20. Stirrat AN, Fyfe IS. Surgery of the rheumatoid cervical spine. Clin Orthop Relat Res 1993;293:135–143
21. Boden SD, Dodge LD, Bohlman HH, Rechtine GR. Rheumatoid arthritis of the cervical spine: a long-term analysis with predictors of paralysis and recovery. J Bone Joint Surg Am 1993;75: 1282–1297
22. Pellicci PM, Ranawat CS, Tsairis P, Bryan WJ. A prospective study of the progression of rheumatoid arthritis of the cervical spine. J Bone Joint Surg Am 1981;63:342–350
23. Ranawat CS, O’Leary P, Pellicci P, Tsairis P, Marchisell P, Dorr L. Cervical spine fusion in rheumatoid arthritis. J Bone Joint Surg Am 1979;61:1003–1010
24. Santavirta S, Konttinen YT, Sandelin J, Slatis P. Operations for the unstable cervical spine in rheumatoid arthritis. Acta Orthop Scand 1990;61:106–110
25. Crockard HA, Grob D. Rheumatoid arthritis: upper cervical involvement. In: Clark CR, ed. The Cervical Spine. Philadelphia: Lippincott-Raven; 1995
26. Sharp J, Purser DW. Spontaneous atlanto-axial dislocation in ankylosing spondylitis and rheumatoid arthritis. Ann Rheum Dis 1961;20:47–77
27. Collins DN, Batnes CL, FitzRandolph RL. Cervical spine instability in rheumatoid patients having total hip or knee arthroplasty. Clin Orthop Relat Res 1991;272:127–135
28. Grauer JN, Tingstad EM, Rand N, Christie MJ, Hilibrand AS. Predictors of paralysis in the rheumatoid cervical spine in patients undergoing total joint arthroplasty. J Bone Joint Surg Am 2004;86: 1420–1424
29. Takahashi M, Yamashita Y, Sakamoto Y, Kojima R. Chronic cervical cord compression: clinical significance of increased signal intensity on MR images. Radiology 1989;173:219–224
30. Bell GR, Stearns KL. Flexion-extension MRI of the upper rheumatoid cervical spine. Orthopedics 1991;14:969–974
31. Kramer J, Jolesz F, Kleefield J. Rheumatoid arthritis of the cervical spine. Rheum Dis Clin North Am 1991;17:757–772
32. Chan DPK, Ngian KS, Cohen L. Posterior upper cervical fusion in rheumatoid arthritis. Spine 1992;17:268–272
33. Gupta S, Goel A. Quantitative anatomy of the lateral masses of the atlas and axis vertebrae. Neurol India 2000;48:120–125
34. Melcher RP, Puttlitz CM, Keinsteck FS, Lotz JC, Harms J, Bradford DS. Biomechanical testing of posterior atlantoaxial fixation techniques. Spine 2002;27:2435–2440
35. Olerud C, Larsson B, Rodriguez M. Subaxial cervical spine subluxation in rheumatoid arthritis. Acta Orthop Scand 1997;68:109–115
36. Omura K, Hukuda S, Katsuura A, Saruhashi Y, Imanaka T, Imai S. Evaluation of posterior long fusion versus conservative treatment for the progressive rheumatoid cervical spine. Spine 2002;27: 1336–1345
37. Oostveen JC, van de Laar M, Geelen J, de Graaff R. Successful conservative treatment of rheumatoid subaxial subluxation resulting in improvement of myelopathy, reduction of subluxation, and stabilization of the cervical spine: a report of two cases. Ann Rheum Dis 1999;58:126–129
38. Mukai Y, Hosono N, Sakaura H, et al. Laminoplasty for cervical myelopathy caused by subaxial lesions in rheumatoid arthritis. J Neurosurg 2004;100:7–13
39. Yoshihiro M, Hosono N, Sakaura H, et al. Laminoplasty for cervical myelopathy caused by subaxial lesions in rheumatoid arthritis. J Neurosurg 2004;100:7–12
< div class='tao-gold-member'>



