Structure of dopamine and rotigotine.
Pharmacokinetics
Rotigotine has a broad spectrum of action across the D1–D5 receptors (Table 6.3) [12]. This, particularly its D1 activity, is in theory an advantage.
Receptor-binding profile of rotigotine
| Affinity for dopamine receptors | Affinity for nondopamine receptors | ||
|---|---|---|---|
| D1 | ++ | 5HT1A | ++ |
| D2 | ++ | α2B | ++ |
| D3 | ++++ | ||
| D4 | +++ | ||
| D5 | +++ | ||
In addition to D3 activity, rotigotine also has considerable affinity for the D1 receptor, unlike other DAs, and this may be important because D1 activity is proposed to synergistically enhance the effect mediated via D2-like receptors [13]. Furthermore, studies suggest that D1 agonism may underlie an antidyskinetic effect as well as a beneficial effect on bladder function, often affected in PD [14].
Rotigotine also binds to serotonin receptors 5-HT1A and 5-HT7, and is an antagonist at α2B receptors. It has been suggested that the action of rotigotine on 5-HT1A and α2B receptors may contribute to other beneficial effects, such as an antidepressant effect (5-HT1A agonistic action) and also antidyskinetic action (α2B antagonistic action) [15, 16]. Furthermore, its lack of affinity for 5-HT2B receptors provides safety in terms of cardiac valvulofibrosis, a problem with ergot-derived DAs.
Rotigotine’s absorption and other pharmacokinetic properties, and also comparison with the other commonly used DAs ropinirole and pramipexole, are shown in Table 6.4 [17].
Pharmacokinetic profile of rotigotine and comparison with ropinirole and pramipexole
| Rotigotine transdermal patch | Ropinirole (oral) | Pramipexole (oral) | |
|---|---|---|---|
| Absorption | |||
| Absorption rate | Linear/24 h | Rapid | Rapid |
| Tmax | Hours | 1–2 h | 2 h |
| Food interaction | None | Tmax: +2.5 h | Tmax: +1 h |
| First-pass effect | None | Yes | Minimal |
| Distribution | |||
| Protein binding | 89.5% | 40% | 15% |
| Metabolism | |||
| Metabolic pathways | Extensive | Extensive | None |
| CYP450 system | Multiple | CYP1A2 | None |
| Active metabolites | None | None | None |
| Elimination | |||
| Renal excretion | 71% | <10% | 90% (tubular secretion) |
| T½ | 5–7 h | 6–8 h | 8–10 h |
In terms of composition, rotigotine is dispersed in a silicone adhesive and then spread evenly across a silicone backing. This permits uniform release of the drug delivery at a constant rate (Figure 6.2). In 2008 concern was raised about crystals developing within the patch which could be avoided by cold storage of the patch [11]. More recently, the need for cold storage of the patch has been reevaluated and the product is now applicable without cold storage.
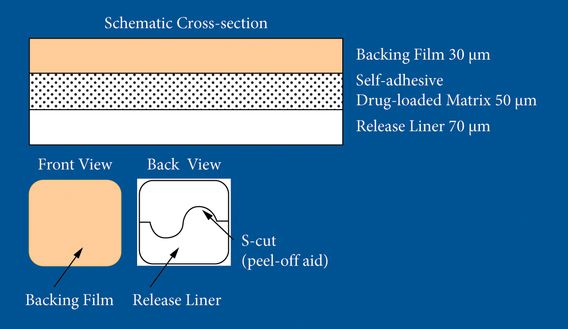
Structure of a rotigotine patch.
Effect on motor function and nonmotor symptoms
Several large, multicenter, randomized, controlled trials, providing a level I evidence base, have demonstrated the safety and efficacy of 24-h RTS for the treatment of motor symptoms associated with both early and advanced PD.
In two placebo-controlled studies [18, 19] transdermal rotigotine, adjunctive to levodopa, showed efficacy for motor complications in advanced PD, as evidenced by significant reductions in “off” time (average 2.5 h) and increases in “on” time without troublesome dyskinesias. In one of the studies [19], a third treatment arm permitted head-to-head comparisons between the rotigotine patch and pramipexole immediate release given three times per day. Even with the rotigotine patch being administered once in the morning, improvements in “off” time, “on” time without troublesome dyskinesias and other outcomes showed no significant differences between these two treatments.
Nonmotor effects
Nonmotor symptoms of PD are a key unmet need, and increasingly clinical trials are required to show nonmotor outcome benefits in addition to motor benefits [6]. A recent large-scale, double-blind, randomized study (RECOVER, Randomized Evaluation of the 24-Hour Coverage: Efficacy of Rotigotine) was the first to investigate early-morning motor function and sleep as co-primary outcome measures in PD [20]. In this study, 24-h transdermal rotigotine treatment was associated with significant benefits versus placebo in terms of early-morning motor impairment and nocturnal sleep disturbances. At 12 weeks, early-morning motor dysfunction as measured by Unified Parkinson’s Disease Rating Scale (UPDRS) III scores and sleep disturbance as measured bythe PD Sleep Scale (PDSS-2) total scores showed significantly greater improvements in the active-treatment group. Difficulty in falling asleep, and feeling tired and sleepy in the morning were among the ten items showing significant improvement. The RECOVER study was also the first large-scale trial to extensively investigate nonmotor symptoms of PD using the Non-Motor Symptoms Scale (NMSS). Significantly greater improvements with rotigotine were seen in NMSS total score, with significant changes on the sleep/fatigue and mood/apathy domains. In addition, the authors reported greater improvements with rotigotine than placebo on depression scores of Beck Depression Inventory (BDI) II, as well as quality of life.
Following the RECOVER study, a post-hoc analysis investigated the effects of rotigotine on individual nonmotor symptoms [21]. This post-hoc analysis suggested that RTS may have a positive effect on fatigue and mood disturbances (symptoms of depression, anhedonia) and apathy in patients with PD.
In another post-hoc analysis of RECOVER, using an 11-point Likert pain scale, authors evaluated the effect of rotigotine on pain [22]. They concluded that pain was improved in patients with PD treated with rotigotine, and this may be partly attributable to benefits in motor function and sleep disturbances.
Further studies addressing the effect of rotigotine patch therapy versus placebo on depression and anxiety are currently under way.
Long-term effect of RTS
The long-term effect of rotigotine in PD was studied in a 1-year study designed as an open-labeled extension of the RECOVER trial. At the end of the maintenance phase, rotigotine was well tolerated; the most common adverse events were application site reactions (24%), somnolence and hallucinations (13% each), nausea (12%), and dizziness and dyskinesias (11% each), most of which were mild or moderate in intensity and resolved at the end of the trial. The beneficial effects of RTS on motor function and sleep disturbances were sustained for up to 1 year [23].
Side effects
Rotigotine is generally well tolerated, and a survey of DA trials suggested that adverse events are similar to those of other DAs, such as nausea, dizziness and somnolence [24]. In clinical trials, the rotigotine transdermal patch was associated with an incidence of application site reaction of 39–44% [25], although only a small subset had a severe reaction. The risk of skin reactions is reduced by daily rotation of the application site, and there is evidence of a lower rate of skin reactions when the rotations are strictly undertaken [25].
Role in impulse control disorders
Impulse control disorders (ICDs) such as compulsive gambling, shopping or hypersexuality are being increasingly recognized in PD patients as adverse effects of DA therapy and pose a major therapeutic challenge [26]. A recent postmarketing observational multicenter European study (the DAICE-ET survey) conducted in Europe has suggested that the risk of developing ICDs may be significantly lower for the rotigotine patch (4.9%) than for shorter-acting agonists, where the rates are considerably higher [27]. The pathophysiological basis of this observation is unclear and may be related to a possible “ICD-sparing” action of the CDD strategy compared with the pulsatile delivery associated with short-acting agents given orally.
Other transdermal DAs
Several other DAs have been developed into a transdermal form, with only apomorphine showing promising results and with a possibility to be used in the future. Two DAs, a lisuride patch and transdermal piribedil, have been discontinued since they failed to show superior efficacy.
Apomorphine transdermal patch
As mentioned above, a transdermal form of apomorphine included in a microemulsion and administered by the transdermal route (Apo-MTD) has also been developed. However, there is only one study showing its effects on PD. Priano et al. [28] studied 21 PD patients who were treated with levodopa plus oral DAs, with levodopa alone or with levodopa plus Apo-MTD. The authors reported steady therapeutic plasma levels, improved UPDRS motor scores and a reduced total duration of “off” periods in the group treated with the Apo-MTD patch. They also concluded that the epicutaneous–transdermal route is able to provide sustained release of the drug and, importantly, therapeutic plasma levels for a period of time that is longer than other DA preparations and is comparable to continuous infusion of apomorphine [28]. However, there have been no further recent developments in this area.
Lisuride transdermal patch
Lisuride patches have been under development for PD in the past. However, they have not been studied extensively because of the drug’s side effects, particularly neuropsychiatric issues. A small open-label study of eight patients showed efficacy and tolerability in the treatment of motor complications in PD [29]. Assessment of “motor changing rate” was performed and it was shown that lisuride patch application significantly (P = 0.023) improved the motor changing rate compared with baseline. The authors noted side effects of transient skin irritations in four patients [29]. Another randomized, placebo-controlled study of 20 PD patients reported (in an abstract form) a significant reduction in motor fluctuations, improved quality of life, decreased daytime somnolence and increased duration of nighttime sleep [30]. Skin irritation was reported as a side effect in this study as well. Currently, therefore, a robust evidence base to support the use of lisuride patches in clinical practice is lacking.
Piribedil transdermal patch
Piribedil is a dopaminergic agonist active on all central dopaminergic pathways, essentially by stimulating postsynaptic D2 receptors [31]. Clinical studies with piribedil administered orally or intravenously have shown its efficacy in PD. However, through the oral route, the drug undergoes a major hepatic first-pass effect and bioavailability is consequently low, at less than 10%. To minimize the hepatic first-pass effect and achieve stable effective plasma concentrations, a 50 mg transdermal patch formulation was developed. However, a single-center, randomized, double-blinded study of 27 PD patients during 3 weeks of treatment administered to three different groups – placebo, one piribedil patch and two piribedil patches – showed no clinical efficacy on the motor symptoms or UPDRS scores [32]. The main adverse events noted in this study were nausea (11%), vomiting (7.4%) and malaise (7.4%); however, these effects were observed mainly in the placebo group (four of seven patients) and the authors reported good local acceptability of the transdermal system. Although the transdermal patch has several advantages (local tolerance, good patch adhesion), it is not currently being actively developed.
Intranasal dopamine agonists
The intranasal administration of solutions has been recognized as a promising route of delivery of therapeutic compounds. It is convenient, with rapid absorption, bioavailability and quality of response for drugs penetrating mucous membranes such as apomorphine.
Apomorphine
Several small open-label studies using intranasal apomorphine were published in the 1990s (Table 6.5) [33–40]. In all of these, intranasal apomorphine improved the motor function of the patients, reducing daily “off” time by 19–50% and improving the motor scores. In two studies, the effect was reported to be comparable to oral levodopa [37] or subcutaneous apomorphine injections [35].
However, in most of the studies, patients experienced significant adverse events. These were not only the common apomorphine side effects such as nausea, vomiting and orthostatic hypotension, but also nasal congestion, crusting and vestibulitis, some of which were severe (in ten cases altogether) and eventually led to discontinuation of the treatment.
Summary of studies using intranasal apomorphine
| Reference | n | Dose | Clinical findings | Adverse events |
|---|---|---|---|---|
| Kapoor et al. (1990) [33] | 8 | 6 mg (0.6 ml) | 50% decrease in off scores | None |
| Kleedorfer et al. (1991) [34] | 5 | 4.3 mg | Decrease of “off” time | Transient nasal congestion Vestibulitis (n = 2) Orthostatic hypotension (n = 4) |
| van Laar et al. (1992) [35] | 7 | 1–10 mg | Improved motor response Similar to s.c. injections | Nasal congestion and crusting (n = 3) Vestibulitis (n = 1) |
| Sam et al. (1995) [36] | 7 | 5.3 mg | Achieved “on” response | Not described |
| Dewey et al. (1996) [37] | 11 | 2–5 mg | Similar to oral levodopa Relieves “off” state | Nausea and vomiting (n = 3), Orthostatic hypotension (n = 1) |
| Esteban Muñoz et al. (1997) [38] | 9 | SC vs intranasal route Reduced daily “off” hours Improved “off” dystonia improved dyskinesias | Nasal crusting Vestibulitis Poor tolerability in intranasal group | |
| Dewey et al. (1998) [39] | 9 | 4.1mg | Improvement in motor scores | Nausea (n = 2) Orthostatic hypotension (n = 2) Nasal irritation (n = 5) |
| Wickremaratchi et al. (2003) [40] | 6 | 5 mg | Induced “on” response | None |
Subcutaneously administered dopamine agonists
Apomorphine infusion and injection
Apomorphine (10,11-dihydroxyapomorphine) is the most potent short-acting DA at D1 and D2 dopamine receptors, and it was proposed as an antiparkinsonian drug more than a century ago. Since apomorphine cannot be absorbed when administered orally, it needs to be given by the subcutaneous route, either intermittently as an injection or as a continuous infusion.
Apomorphine was first used by veterinary physicians in animals to treat behavioral vices before its emetic properties were exploited as a treatment for poisoning. At high doses, apomorphine caused involuntary movement and stereotyped behavior similar to punding, but at lower doses it had a robust beneficial motor effect and this was evidence that different doses of apomorphine could produce different outcomes in the context of PD [41].
Schwab and colleagues in the USA were the first to demonstrate the antiparkinsonian properties of apomorphine in 1951 [42] and showed that apomorphine relieved rigidity and tremor for periods of up to 30–40 min. Subsequently, Cotzias et al. [43] suggested that apomorphine improved tremor refractory to dopamine and may have an antidyskinetic effect.
The development of ambulatory mini pumps for the management of brittle diabetes mellitus and use of the peripheral dopamine antagonist domperidone were utilized to define the role of apomorphine single injections as a “rescue” therapy for patients with predictable “off” periods. In the UK, work from the group of Lees and colleagues and others showed that continuous waking day subcutaneous apomorphine delivered by a GrasebyTM dynamics pump resulted in a dramatic reduction in the frequency and duration of “off” periods (a reduction of 10 h “off”/day to 3–4 h “off” per day) [44, 45]. These data suggest that apomorphine, to this day, is the only clinically available DA that is equipotent to levodopa.
Currently, apomorphine continuous infusion is generally used when continuous dopaminergic stimulation is required in PD patients with increasing or prolonged “off” periods, motor fluctuations and/or moderate to severe levodopa-induced dyskinesias. Apomorphine can now be delivered via a small, portable pump (APO-go® pump) worn by the patient using a low-impact NeriaTM line for subcutaneous delivery (Figure 6.3).
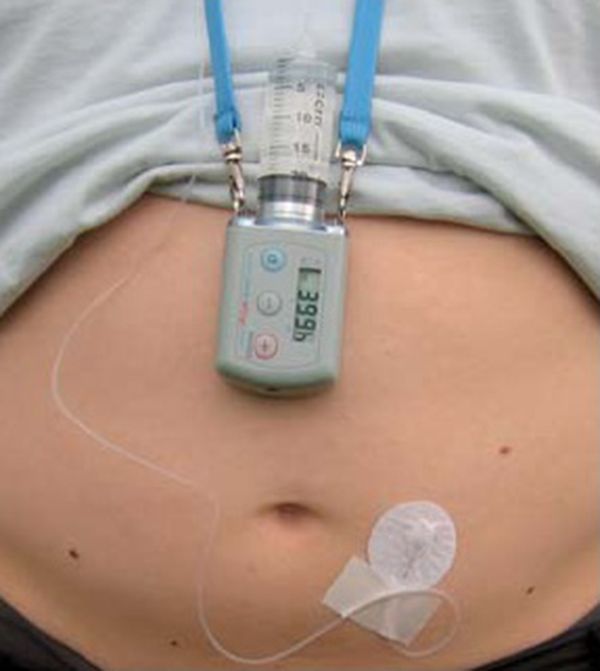
Patient with an apomorphine pump.
A portable pump permits continuous infusion of the drug into the subcutaneous fatty tissue of the abdomen, thighs or arms [46], and in Europe and parts of Asia and Australia, this specific delivery method using NeriaTM lines is available. After long-term usage, however, inflammatory skin nodules may form, which may interfere with drug absorption [47], and active and continuing skin care is an essential part of apomorphine therapy (Table 6.6).
Indications for using an apomorphine pen or pump
| Pen | Pump |
|---|---|
|
|
Pharmacokinetics of subcutaneous apomorphine
The pharmacokinetics of apomorphine are shown in Table 6.7.
Pharmacokinetic profile of apomorphine
| Rapidly absorbed and correlates with rapid onset of clinical effect |
| Peak levels achieved after 3–5 min |
| Absorption of apomorphine varies among patients (5–10-fold difference) |
| Half-life in distribution phase of ∼5 min |
| Biological half-life in elimination phase of ∼33 min |
| Brief clinical effect is accounted for by the rapid clearance of apomorphine |
Effect on motor complications: dyskinesias and “off” periods
In a range of clinical trials, apomorphine has been shown to have equivalent efficacy to oral levodopa [41]. The injection formulation comprises apomorphine hydrochloride 10 mg/ml for subcutaneous injection in a multidose, disposable pen. This formulation is intended for use by PD patients who are experiencing refractory periods, which occur when oral medications start to fail (see Table 6.6). It is recommended as a rescue treatment for those patients experiencing only a few “off” episodes per day, as an adjunct to oral medications. Apomorphine injection has a rapid onset (4–12 min) and a duration of action of about 1 h. In an open-label study, PD patients were switched from subcutaneous to intravenous apomorphine (delivered by indwelling venous catheter) in patients with refractory motor fluctuations and severe dyskinesias [48]. There was a dramatic reduction in dyskinesias with virtual complete elimination of the “off” period (mean reduction from 5.4 to 0.5 h; P < 0.05); however, severe and serious side effects including cardiovascular ones resulted in discontinuation of the study. In this study, plasma apomorphine levels did not correlate well with dosage level or with motor function and response. However, in a small study of two PD patients, plasma apomorphine levels again showed weak correlation with motor function, although cerebrospinal fluid apomorphine levels showed a strong correlation with motor function [49]. Clinical studies addressing the effects of apomorphine in PD are shown in Table 6.8.
Summary of studies (mostly open label) using apomorphine infusion in Parkinson’s disease
| Reference | n | Follow-up period (months) | Daily time in “off” period (%) | Dyskinesia intensity (%) |
|---|---|---|---|---|
| Stibe et al. (1988) [44] | 11 | 8 | −62 | |
| Chaudhuri et al. (1988) [45] | 7 | 11 | −85 | −45 |
| Frankel et al. (1990) [50] | 25 | 22 | −55 | |
| Pollak et al. (1990) [51] | 9 | 10 | −67 | −20 |
| Hughes et al. (1993) [52] | 22 | 36 | −59 | |
| Stochhi et al. (1993) [53] | 10 | 12 | −58 | −40 |
| Poewe et al. (1993) [54] | 18 | 20 | −58 | |
| Kreczy-Kleedorfer et al. (1993) [55] | 14 | 26 | −77 | |
| Gancher et al. (1995) [56] | 7 | 3 | −58 | |
| Colzi et al. (1998) [57] | 19 | 35 | −72 | −65 |
| Pietz et al. (1998) [58] | 25 | 44 | −50 | −14 |
| Wenning et al. (1999) [59] | 16 | 57 | −55 | |
| Stocchi et al. (2001) [60] | 30 | 60 | ||
| Kanovsky et al. (2002) [61] | 12 | 24 | −80 | |
| Manson et al. (2002) [62] | 64 | 34 | −49 | −57 |
| Di Rosa et al. (2003) [63] | 12 | 12 | −40 | −37 |
| Morgante et al. (2004) [64] | 12 | 24 | −60 | −48 |
| Tyne et al. (2004) [65] | 80 | 25 | ||
| Katzenschlager et al. (2005) [66] | 12 | 6 | −38 | −31 |
| De Gaspari et al. (2006) [67] | 13 | 12 | −51 | No change |
| Garcia-Ruiz et al. (2008) [68] | 82 | 20 | −80 | −32 |
| Martinez-Martin et al. (2011) [69] | 17 | 6 | −65 | |
| Antonini et al. (2011) [70] | 12 | 60 | −49 | No change |
| Drapier et al. (2012) [71] | 23 | 12 | −36 | |
| Summary (total number of patients; mean reduction of time in “off” period and dyskinesia scores) | 552 | −59.3 | −32.4 |
Several long-term, open-label, uncontrolled studies evaluated the efficacy of continuous subcutaneous apomorphine infusions in monotherapy or as an add-on to levodopa therapy in advanced PD (Table 6.8) [44, 45, 52, 54, 57–62, 66]. These studies showed that subcutaneous apomorphine infusions are successful in aborting “off” periods (often not amenable to oral treatment), reducing dyskinesias and improving PD motor scores [41, 57, 62, 66, 68].
Most of the studies [44, 52, 54, 58, 59] reported mainly large reductions (50–72%) in daily “off” time in patients treated with continuous apomorphine infusion (as in Table 6.8). In addition, a marked and sustained reduction (43–64%) in dyskinesias has been reported in those patients who achieve a substantial reduction in their oral dopaminergic therapy [57, 60–62, 66, 68]. However, this observation has not been supported in two open-label, nonrandomized studies and a 5-year prospective study that compared apomorphine with deep-brain stimulation of the subthalamic nucleus [67, 70, 72]. While a similar efficiency in reducing “off” time (51–76%) was observed in both groups, dyskinesia duration and severity was reduced only in the deep-brain stimulation group.
Nonmotor effects of apomorphine
While there is a reasonable body of evidence that confirms the efficacy of apomorphine on motor function in PD, as well as a possible antidyskinetic effects [45], there have also been reports of the possible beneficial effect of apomorphine on nonmotor symptoms in PD stretching back to Cotzias suggesting an antipsychotic effect. Given that nonmotor symptoms are now recognized to be almost universal across all stages of PD [6] and are also the key determinant of quality of life of people with PD [69], a closer examination of the nonmotor effects of apomorphine is justified. In a recent review, Todorova and Chaudhuri [73] produced a summary of the possible beneficial nonmotor effects of apomorphine in PD.
A controlled comparative study (not blinded and nonrandomized) addressing apomorphine and nonmotor symptoms was performed by Martinez-Martin et al. [69] using the NMSS as one primary outcome variable in addition to standard motor outcomes. The authors compared 17 patients receiving subcutaneous apomorphine infusion with a patient group on conservative therapy with motor, nonmotor and quality-of-life measures being assessed at initiation of therapy and at a 6-month follow-up as part of the routine clinical practice in a real-life study. Apomorphine infusion was found to improve NMSS total score significantly (P = 0.0003), quality of life as measured by the 8-Item Parkinson’s Disease Questionnaire (PDQ-8) and motor state as measured by UPDRS-III and -IV scores. When the NMSS domains were subanalyzed, moderate to large improvements in effect size of apomorphine were observed in sleep (mainly related to nocturnal restless legs-type symptoms and insomnia), attention, mood, apathy, fatigue, urinary (urgency, nocturia) and gastrointestinal (dribbling, constipation) domains of the NMSS [69]. Importantly, quality-of-life scores deteriorated significantly in the group maintained on optimal oral therapy versus the apomorphine group where a significant benefit in quality of life was observed.
Some have argued that the piperidine moiety contained in the structure of apomorphine may have some antipsychotic properties. In specific studies addressing this issue, Chaudhuri et al. [74] and Ellis et al. [75] published a total of 15 case reports on PD patients with previous drug-related neuropsychiatric problems, confirming the possible role of the use of apomorphine in PD patients with neuropsychiatric problems. Contrary to common perception, apomorphine therapy can be tolerated in patients experiencing visual hallucinations and delusions, as well as in patients with paranoid ideations [69]. Apomorphine infusion was well tolerated in PD patients experiencing hallucinations [75, 76]. Some authors have even suggested that apomorphine may improve visual processing in PD patients with visual hallucinations, in particular increasing contrast sensitivity and decreasing reaction time [76]. These observations have never been studied in a controlled fashion and thus remain controversial.
Gastroparesis is emerging as a key problem in PD, both early and advanced [77], and there is evidence that nonoral therapies such as apomorphine infusion or a rotigotine patch in this scenario are particularly effective. Preliminary data (shown in an abstract form) from the AM IMPAKT (Apokyn for Motor Improvement of Morning Akinesia Trial) study (a phase 4, multicenter, open-label study to investigate the rapid and reliable improvement of motor symptoms with APOKYN® in PD patients with morning akinesia resulting from delayed or unreliable onset of levodopa action) showed that apomorphine provided rapid and reliable turning on for patients with morning akinesia, and had a significant impact on PD patients who experience delayed “on” to levodopa in the early morning.
In relation to other gastrointestinal symptoms, two studies have reported on defecatory dysfunction, and in both studies, apomorphine showed a positive effect on anorectal dysfunction in PD [78, 79]. Tison et al. [80] studied the effects of apomorphine on liquid swallowing using videofluoroscopyand found that apomorphine improved swallowing abnormalities in a subgroup of PD patients with swallowing disorders.
There are only a few case reports on apomorphine in restless legs syndrome (RLS). In a patient with idiopathic RLS and another with PD and RLS, an overnight infusion with apomorphine improved nocturnal discomfort and leg movements [81], and resulted in a rapid and significant (55%) improvement in subjective RLS symptoms and an almost immediate cessation of periodic limb movements [82].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



