, Nima Etminan1 and Daniel Hänggi1, 2
(1)
Neurochirurgische Klinik, Universitätsklinikum Düsseldorf, Düsseldorf, Germany
(2)
Medical Art Christine Opfermann-Rüngeler, Zentrum für Anatomie Heinrich Heine Universität, Düsseldorf, Germany
4.1 General Philosophy and Procedures
4.1.1 Approaches
In recent decades, the philosophy regarding approaches and adjuvant measures (i.e., imaging and planning tools and neurophysiological monitoring) has seen considerable changes. Following its formal description in the 1960s and 1970s, the pterional approach became standard for most anterior circulation aneurysms [1] and served well for some 30 years. It was a more precise and limited opening than the formerly used generous frontotemporal craniotomy. The success of the pterional craniotomy would not have been possible without the simultaneous introduction of the surgical microscope.
The advent of endovascular techniques, however, called for less invasive surgical procedures, as patients with the choice of less traumatic alternatives no longer accepted the traditional “big” openings. Further reduction of the craniotomy size was only possible by giving up the concept of one standard craniotomy for the principal anterior circulation aneurysms. Smaller craniotomies must be placed optimally and centered on the most direct path to the neck of the aneurysm. Moreover, the approach needs to allow safe control of the parent arteries. Although the exposure of the aneurysm and the parent arteries should be done in a manner that is focused on the pathology, safety and control are the principal rules for aneurysm surgery. Minimally invasive approaches must never be narrow and confining. Otherwise, disaster in the event of intraoperative aneurysm rupture is unavoidable. Minimizing the craniotomy potentially may compromise safety by reducing control of the parent artery, by limiting the view of perforating branches, and by restricting the degrees of freedom for surgical manipulation. This potential conflict must be kept in mind, as the safety of the procedure must be seen as the first priority. In our experience, a diameter of less than 4 cm is too small for general application, though smaller craniotomies can be considered exceptionally for certain unruptured aneurysms.
Prior to surgery, a master plan of the craniotomy and the approach to the aneurysm should be made, identifying the most direct route to the aneurysm that requires minimal retraction and minimal microsurgical dissection. In our experience, it is important to discriminate between aneurysms accessible through basal approaches (e.g., aneurysms of the carotid artery) and aneurysms accessible through hemispheric approaches (e.g., middle cerebral artery [MCA] and pericallosal artery aneurysms). The orientation and the exact placement of a small craniotomy for aneurysms reachable through approaches near the skull base are facilitated by the presence of enough anatomical landmarks, whereas a hemispheric approach is better performed with the help of navigation.
We use four different tailored craniotomies for the aneurysms of the anterior circle of Willis (Fig. 4.1): the orbitocraniotomy for aneurysms of the anterior communicating artery, the pterional craniotomy for aneurysms of the internal carotid artery, the Sylvian craniotomy for the MCA aneurysm, and a small interhemispheric craniotomy for distal aneurysms of the anterior cerebral artery (i.e., at the origin of the callosomarginal artery). For aneurysms of the posterior circulation that are addressed by microsurgery (i.e., vertebral artery–posterior inferior cerebellar artery (PICA) aneurysms and peripheral aneurysms of the cerebellar arteries), either a lateral paracondylar targeted craniotomy or a paramedian opening is used.
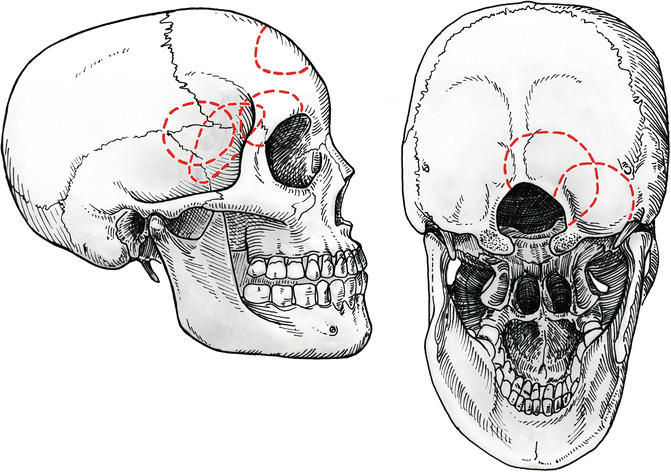

Fig. 4.1
Four different tailored craniotomies are used for typical anterior circulation aneurysms: pterional craniotomy for internal carotid aneurysms, Sylvian craniotomy for middle cerebral aneurysms, orbitocraniotomy for anterior communicating artery, and anterior interhemispheric craniotomy for A2-callosomarginal artery aneurysms. Two approaches are used for posterior circulation aneurysms: lateral paracondylar for vertebral artery aneurysms and paramedian for peripheral aneurysms
4.1.2 CSF Drainage
Brain retraction should be avoided as far as possible. Brain relaxation is achieved by cisternal, ventricular, or spinal drainage of cerebrospinal fluid (CSF) and by administering mannitol, if necessary. We use a spinal or ventricular catheter for all aneurysm operations in the acute stage after subarachnoid hemorrhage (Fig. 4.2). Lumbar spinal drainage is preferred for good-grade patients (World Federation of Neurosurgical Societies [WFNS] I or II), and ventricular drainage is preferred for WFNS grades III to V. In these latter patients, the ventricular catheter is routinely inserted at the time of admission via a frontal burr hole at Kocher’s point. In order to avoid stress, this procedure is done under general anesthesia and the patient remains deeply sedated and intubated until the aneurysm has been secured surgically or by endovascular coiling.
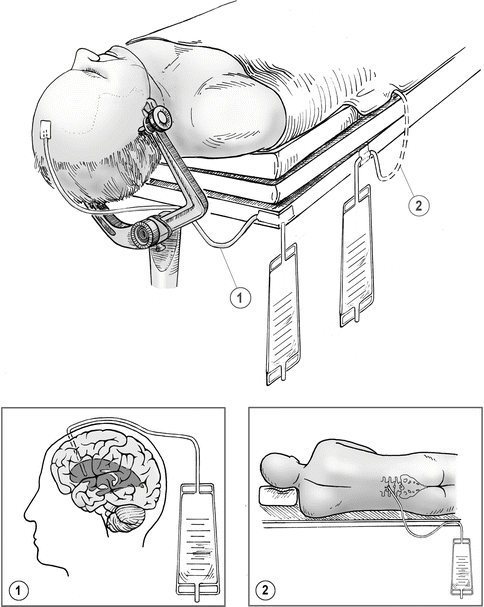

Fig. 4.2
Either ventricular (1) or spinal (2) cerebrospinal fluid (CSF) drainage is used for all patients with ruptured aneurysms
Space-occupying hematomas are an exception; initial ventricular drainage is contraindicated for these patients because of the time lost and the substantial risk of rerupture. Efficient craniotomy and evacuation of the hematoma is the quickest and most efficient way to decrease intracranial pressure in these cases.
In good-grade patients, the lumbar catheter is inserted after induction of general anesthesia for the aneurysm procedure and removed at the end of the procedure.
4.1.3 Intraoperative Monitoring
In contrast to brain tumor surgery, electrophysiological monitoring and navigation have not been generally accepted for aneurysm surgery. The reasons are explained by the nonelective nature of most aneurysm procedures. Electrophysiological monitoring cannot prevent all ischemic complications. For example, when losing a parent artery or perforating branch, the warning signs from monitoring are of little consequence. On the other hand, electrophysiological monitoring can recognize the inadvertent occlusion of an arterial branch [2]. In this situation, revision of clip positions may solve the problem and prevent permanent damage, so we recommend inclusion of electrophysiological monitoring whenever possible.
4.1.4 Neuronavigation
With regard to the use of navigation, things are less clear [3, 4]. It is important to use the angiographic 3D reconstruction for planning the approach and clip application. It is best to rotate the 3D reconstruction so that the view corresponds closely to the intraoperative view. The limited degrees of freedom with the tailored small craniotomies make good planning mandatory. Neuronavigation adds little benefit for the anterior communicating and internal carotid artery aneurysm, but it is helpful for the more variable locations. The benefit for MCA aneurysms is limited, but there is little doubt that the benefit for distal aneurysms of the anterior cerebral artery (i.e., at the origin of the callosomarginal artery) is real. For peripheral aneurysms of all vascular territories (i.e., mycotic aneurysms), the use of navigation is clearly advantageous.
4.1.5 Positioning
Surgical positioning deserves a great degree of attention and needs to be seen mainly in the context of approach planning. General principles to prevent pressure lesions and cranial venous stasis clearly need to be respected, but positioning also needs to be ergonomically optimized to allow the surgeon to work in a natural posture.
4.2 Orbitocraniotomy for Anterior Communicating Artery Aneurysms
We introduced orbitocraniotomy as the standard surgical approach for anterior communicating artery aneurysms some two decades ago, looking for a more ventral line of access and thus avoiding necessary brain retraction [5, 6]. The approach has proved to be safe and effective.
4.2.1 Typical Indications
Anterior communicating artery aneurysms
A1 aneurysms
4.2.2 Anatomical Landmarks
Frontal sinus
Zygoma
Orbital ridge
Frontal nerve
Orbital roof
Olfactory bulb
Interhemispheric fissure
4.2.3 Positioning and Skin Incision
After induction of endotracheal anesthesia, a spinal drainage catheter is inserted in all good-grade patients operated on acutely after subarachnoid hemorrhage. Ventriculostomy is inserted at the time of admission in patients admitted in WFNS grade IV or V.
The patients are placed in a supine position with the head rotated 45° to the side opposite the planned craniotomy (Fig. 4.3). All anterior communicating artery aneurysms are approached principally from the side of the dominant A1 segment. Approaching from the side of the dominant A1 segment allows for control of the aneurysm with minimal dissection and manipulation of adjacent cerebral structures. The 45° initial rotation represents an average starting point. Occasionally, a more lateral or a more anterior view is necessary during surgery. Fine adjustments of the head rotation during surgery are accomplished by tilting the operating table. The neck of the patient is moderately hyperextended, resulting in an angle of 10–20° between the plane of the anterior cranial fossa and the vertical plane. This position allows the orbital cortex to fall away from the orbital roof, thus minimizing the need for brain retraction. With 45° of head rotation, it is generally not necessary to use a cushion to support the ipsilateral shoulder. A shoulder support may be required in selected patients with degenerative changes of the cervical spine and consequent limited range of motion.
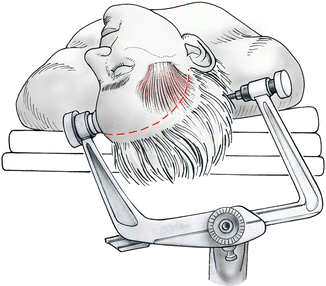

Fig. 4.3
Positioning with the head rotated some 45° and moderately hyperextended
The head is secured in a Mayfield clamp, with the clamp positioned as horizontally as possible. During adjustment and balancing of the surgical microscope, care should be taken that the neutral baseline position of the objective tube is set at an angle 20° from vertical. This position allows easy manipulation of the microscope within the entire range of motion necessary for the orbitocranial approach.
We recommend a temporofrontal hairline skin incision, although an eyebrow incision is a viable alternative. The skin incision is started 1 cm in front of the tragus and slightly above the zygomatic arch. After a cautious incision has been made in the skin and the galea, the superficial temporal artery within the temporal fascia is identified. The frontal or parietal branch of the artery is divided if necessary, and a small hemoclip is used to secure the proximal stump. The scalp incision is extended to the frontal midline.
4.2.4 Soft Tissue Dissection
The skin flap is gently elevated and turned back. Dissection is performed in the interfascial plane between the temporal fascia and the aponeurosis of the temporal muscle. The supraorbital fat pad must be mobilized together with the scalp flap to prevent injury to the frontal branch of the facial nerve. The anterior attachment of the temporal muscle at the orbital pillar and the anterior aspect of the linea temporalis are incised sharply. The temporal muscle is then dissected from the underlying bone and pulled back using a galea hook (Fig. 4.4). The temporal muscle should not be mobilized more than absolutely necessary for the limited craniotomy. The lateral and superior orbital rims are then dissected from the periorbita using a blunt curved dissector. At this stage, attention should be paid to the frontal nerve as it turns around the orbital rim at the medial aspect of the planned craniotomy. Occasionally, it may become necessary to mobilize the frontal nerve out of its bony groove or tunnel.
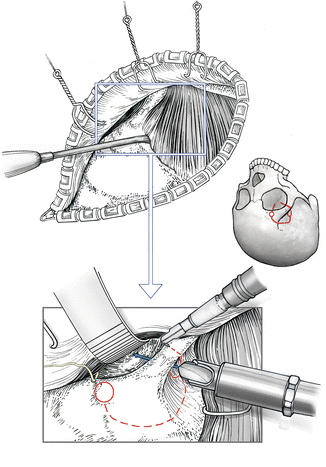

Fig. 4.4
Detachment of the anterior temporal muscle. Outline of orbitocraniotomy, which starts from two burr holes. The lateral burr hole is placed at the orbitozygomatic junction as the keyhole for the pterional craniotomy. In contrast to the pterional craniotomy, the axis of the burr is pointed more toward the orbit, so that the anterior cranial fossa and the orbit are opened simultaneously
4.2.5 Orbitocraniotomy
We use two burr holes for the craniotomy. The initial burr hole corresponds to the keyhole of the pterional craniotomy placed at the frontozygomatic suture. The direction of the burr hole, however, aims more toward the orbit so that the orbit and the anterior cranial fossa can be opened with this single burr hole (see Fig. 4.4). A second burr hole is then placed above the upper orbital rim at the medial aspect of the planned craniotomy. The dura is separated from the orbital roof and the convexity with the use of a blunt dissector. The two burr holes are then connected along the planned upper delineation of the orbitocraniotomy by using the craniotome (Fig. 4.5). A small bone saw is used to cut the superior and lateral orbital rims. The dura and the periorbita must be protected with a small spatula. The bone cut in the lateral orbital rim is extended down to the lateral burr hole. At the medial burr hole, the orbital roof must be cut as far down as possible. The posterior aspect of the medial orbital roof is divided through the medial burr hole with a small punch or a diamond drill. At the lateral burr hole, the orbital roof is divided along the pterion to a depth of 3–4 cm with the use of a small punch. The bone flap is then elevated, and the orbital roof is fractured between the end of the medial and lateral orbital roof incisions. Care should be taken to place the fracture line as far back as possible. We do not advocate removal of the residual orbital roof in an osteoclastic manner after the bone flap is turned. In that event, the orbital roof must be reconstructed later with foreign material to prevent orbital roof instability with enophthalmos or pulsating exophthalmos.
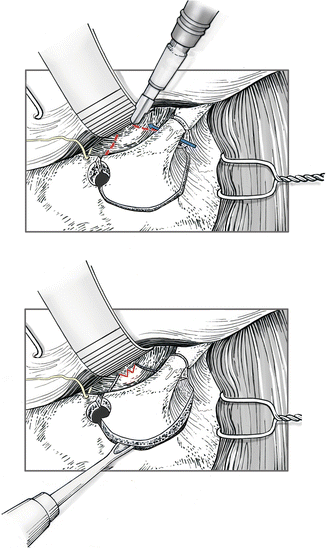

Fig. 4.5
The burr holes are then connected through the frontal bone by using a craniotome. The medial and lateral orbital rims are cut with a small blade saw. The lateral bone cut is extended down to the lateral burr hole. The lateral and medial aspects of the orbital roof are divided through the burr holes with the blade saw or a punch. The bone flap is then elevated, and the orbital roof is fractured as far toward the apex of the orbit as possible
4.2.6 Dural Opening and Approach
A dural flap is created in a curvilinear fashion and reflected basally. The dural incision does not cross the Sylvian fissure. After the dura is opened, approximately 50–100 mL of CSF is drained via the spinal drainage or the ventriculostomy. The orbital cortex is gently elevated. At this stage, the central aspect of the Sylvian fissure is visualized. This central aspect of the Sylvian fissure is carefully split in the ordinary manner down to the internal carotid bifurcation (Fig. 4.6). The posterior aspect of the gyrus rectus is then separated from the optic chiasm. The A1 segment usually is controlled at this time. The next critical step is to split the interhemispheric fissure. The superficial arachnoid layer is opened by using a small microhook. The interhemispheric arachnoid adhesions in the deeper layers can be separated by using the bipolar forceps in a spreading motion such as that usually performed at the level of the Sylvian fissure. The projection of the aneurysm dome must be taken into account during this stage. At this point, the posterior aspect of the gyrus rectus has been freed completely and can be mobilized to provide access to the A1–A2 junction and the anterior communicating artery. The exposure is stabilized by using a small spatula. To prevent damage to the orbital cortex, the brain is supported at the lateral and medial border of the craniotomy with a rolled cotton pledget to distribute the pressure on the frontal cortex. Occasionally, the projection of the aneurysm fundus renders formal splitting of the interhemispheric fissure inappropriate. In this situation, a small medial reduction of the gyrus rectus must be performed. However, it is always possible to limit the resection to the portion of the gyrus rectus medial to the olfactory tract.
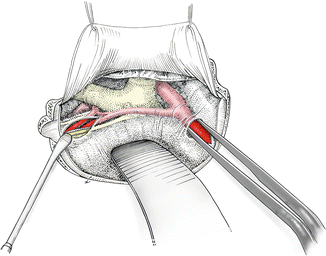

Fig. 4.6
Following dural opening, the central aspect of the Sylvian fissure is opened, as well as the suprachiasmatic cistern and the interhemispheric fissure
4.2.7 Closure
The dura is closed in standard watertight fashion. The periorbita is injured occasionally during dissection from the orbital roof. To prevent protrusion of the orbital fat, periorbital tears should be repaired immediately with a suture. If the frontal sinus has been opened at the medial aspect of the opening, the defect is plugged with a muscle graft taken from the temporalis muscle. We do not recommend use of a pediculated periosteal flap with the orbitocraniotomy, because it risks interference with eyelid and eyebrow motility. The bone flap is repositioned and attached by using small titanium platelets. The anterior aspect of the temporalis muscle is reattached at the linea temporalis. For this purpose, two tangential burr holes are made in the bone flap, and the muscle is reattached with two 3–0 sutures.
4.2.8 Pitfalls and Complications
Dural and periorbital laceration
Frontal nerve injuries
CSF fistula due to insufficient plugging of the frontal sinus and dural closure
Pressure injuries to orbital contents
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








