Figure 35.1. Temporal epidural hematoma displaying the typical biconvex lens appearance.
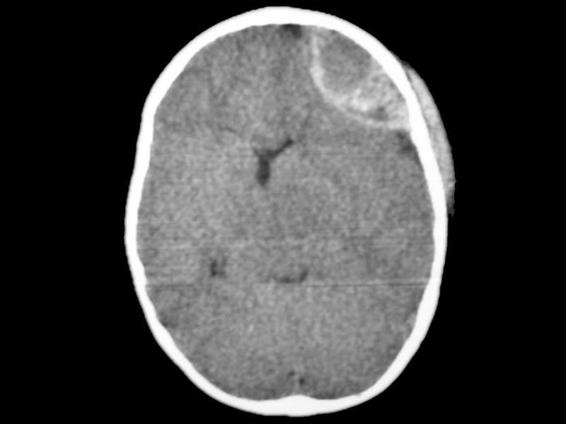
Figure 35.2. Frontal epidural hematoma in the hyperacute phase; note the hypodense areas inside the collection of blood typical of the hyperacute phase.
35.2.2 Surgical Treatment
Most epidural hematomas must be treated surgically (Figure 35.3 A and B). Critical is the initial management in the emergency room and at the scene of the accident, as well as rapid specialist evaluation once the patient is stabilized. In patients presenting with progressive neurological deterioration it is very important to avoid secondary damage and to provide adequate brain resuscitation when needed. If the patient cannot be adequately stabilized and no other reason is found that justifies the neurological condition, or another studies are delayed as may happen in centres lacking available clinical services, urgent surgical evacuation as a salvage manoeuvre may be indicated [1].
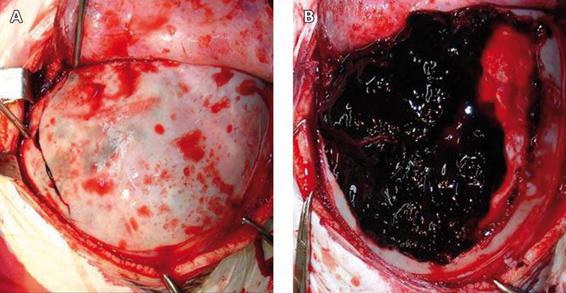
Figure 35.3. Intraoperative image of an epidural hematoma. The fracture on the posterior temporal region can be seen, with protrusion of the hematoma through the fracture (A). A huge epidural hematoma in the same patient after craniotomy (B).
We can use the side of pupil dilatation, the side opposite the motor deficit or even the side of the fracture on the x-ray film to determine hematoma location in 85% of cases. In stabilized patients, or those without progressive neurological deterioration, all necessary studies should be completed. Bone resection for hematomas in the temporal region should be performed up to the floor of the medial fossa if it is necessary to gain access to the proximal portion of the medial meningeal artery when bleeding cannot be controlled. Intracranial pressure monitoring is not necessary if there are no associated parenchymal lesions. There are reports of medical management for epidural hematoma. In most of these patients not treated surgically without clinical manifestations or any neurological deficit, the lesion volume is <40 ml. Such management entails prolonged hospitalization and serial CT scans to evaluate lesion resolution.
Prognosis is related to the level of consciousness, the presence of other neurological deficits and the severity of associated intracranial lesions. In patients admitted not in coma the outcome is favourable in 90-100% of cases and the mortality is 0-5%. In patients with a Glasgow Coma Scale (GCS) score <8 the outcome is favourable in 38-73% of cases and the mortality is 11-41%. The risk of mortality is three times higher if there are associated lesions (subdural hematoma or contusions).
Prompt diagnosis and surgical evacuation are key to achieving a good outcome if there are no associated lesions and neurological deterioration is related only to hematoma expansion. Guidelines for the management of these lesions are summarized in Table 35.1.
Indications for surgery |
|
Timing |
|
Type of surgery |
|
Table 35.1. Guidelines for the surgical management of epidural hematoma.
35.2.3 Non-surgical Management
There are reports of spontaneous resolution of epidural hematoma. This has been explained by reabsorption of the hematoma by the fibrovascular membrane over a period of 13 days. An epidural hematoma may increase in size during this time. Epidural hematomas that can be successfully managed with this criterion are those with a volume <44 cm3. Special care must be taken if the lesion is located in the posterior fossa or the middle fossa owing to the possibility of temporal lobe herniation due to brain swelling and brainstem injury. Any sign of neurological deterioration or signs and symptoms of increased intracranial pressure is an indication for surgical evacuation.
35.2.4 Prognosis
Global mortality is 0-10% but depends on the neurological state at the time of surgery. The mortality rate for comatose patients at surgery is 14-70%. Outcome is usually worse in patients older than 40 years or with lesions >150 cm3 in volume
35.3 Subdural Hematomas
Subdural hematoma is an accumulation of blood between the dura and the brain. Road accidents are the most common cause. A subdural hematoma may be simple or complex. The simpler ones are not accompanied by parenchymal lesions and are usually caused by laceration of the bridging veins between the brain and the dural sinuses. Urgent evacuation is associated with good prognosis. Complex subdural hematoma is associated with parenchymal contusions and high intracranial pressure. Generally, it is located on the temporal, frontal or orbitofrontal lobe and the convexity in the interhemispheric space. The external margin is determined by the skull and the internal margin by the brain, which lends the margin its irregular shape. The volume may increase because of the lack of adherences on the subdural space.
But blood accumulation is not the only cause related to poor prognosis. Brain swelling and ischemia usually associated with subdural hematoma may be related to blood derivates and their neurotoxic action. This ischemic condition may not change with hematoma evacuation. The presence of this lesion justifies urgent surgical evacuation.
35.3.1 Clinical Manifestations
Signs and symptoms result from the mass effect of subdural hematoma, elevated intracranial pressure, location, size, association with another space-occupying lesions, and secondary insults. Early seizures are frequent (24%). Pupillary abnormalities may be seen, as well as motor deficits (<50% of patients); when present they may help localize the side of the lesion. But it should be kept in mind that in 25-30% of cases pupillary signs and in 38% of cases motor signs may give a false localization, which is why CT scan is of vital importance in the management of these patients.
35.3.2 Diagnosis
CT scan without contrast and with bone window view is the study of choice. The CT scan will show a concave-convex lens next to the skull (Figure 35.4) generally over the parietal, temporal and frontal lobes and usually unilateral or occasionally bilateral (15% of cases). Subdural hematomas extend beyond the bone suture line, which helps to differentiate them from epidural hematoma. In 66-80% of cases subdural hematoma is associated with other intracerebral lesions (contusions, hematomas); it may be seen in a hyperacute state with characteristics resembling those of epidural hematoma and it may also be related to coagulation disorders. Interhemispheric subdural hematoma is uncommon.
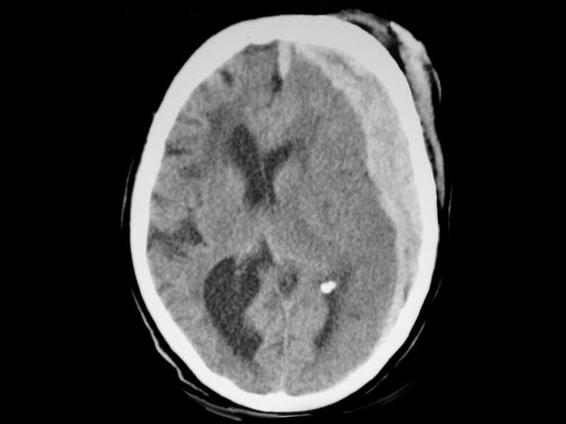
Figure 35.4. Fronto-temporo-parietal subdural hematoma, also known as hemispheric, with the shape of a biconcave lens and hyperacute content characteristic of a hyperacute lesion.
35.3.3 Surgical Treatment
Factors to be weighed before operating on a subdural hematoma are the size, timing (time since the CT scan was performed and time elapsed since the trauma), presence of associated lesions and brain swelling [2].
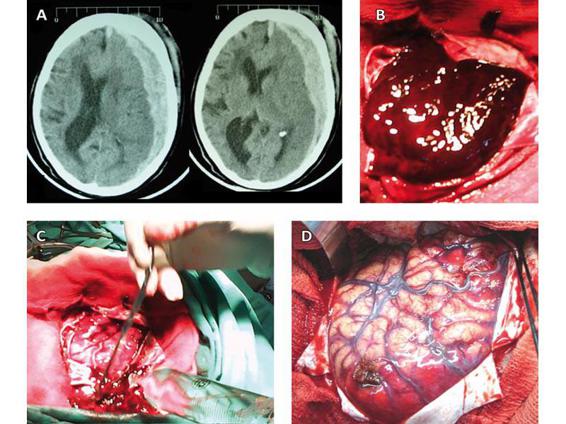
Figure 35.5. CT scan showing an acute hemispheric subdural hematoma with mass effect, midline shift and collapse of the subarachnoid space (A). Intraoperative image showing the large hematoma immediately after dural opening (B). Manoeuvres of washing and aspiration to achieve complete removal of the hematoma (C). Swelling, areas of contusion, and traumatic subarachnoid hemorrhage (TSH) of the brain after lesion evacuation (D).
Subdural hematoma carries a poorer prognosis than epidural hematoma. Prompt evacuation in the first 4 hours seems to be related to better outcome. The delay in surgical evacuation once the decision is taken to operate does not offer any advantage, which is why the procedure should be done urgently (Figure 35.5).
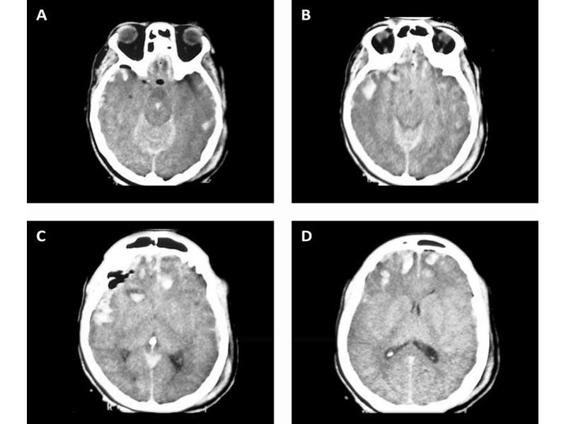
Figure 35.6. Multiple bifrontal contusions and left temporal with TSH.
Subdural hematoma <3 mm in diameter could be observed; but in patients with a GCS score <8 ICP monitoring is indicated with continuous follow-up by the neurosurgical team.
Unquestionable indications are those with clear neurological deterioration, presence of mass effect on the CT scan, intracerebral bleeding, compression of basal cisterns or midline shift >5 mm. There are patients in which the midline shift is considerably greater than expected as compared to the hematoma width. This should prompt us to consider the possibility of brain swelling or adjacent brain injury (infarction). Mortality increases the greater the difference between midline shift and hematoma width. Guidelines for the management of acute subdural hematoma are summarized in Table 35.2.
Indications for surgery |
|
Timing |
|
Type of surgery |
|
Table 35.2. Guidelines for the surgical management of subdural hematoma.
35.3.4 Postoperative Complications
The most common complications are hematoma recurrence, infection and intracerebral hematomas. The recurrence may be subdural or epidural or combined, the last of which is associated with coagulation disorders. Infections develop in <5% of cases. Increased ICP may be present in 50% of cases, which is why ICP monitoring is indicated.
35.3.5 Nonsurgical Management
Surgical evacuation is suggested for treating hematomas >4 mm in width. Smaller collections may resolve spontaneously. It is believe that the slow and progressive spread over the brain surface may help the hematoma to dissolve or to mix with the cerebrospinal fluid. In comatose patients with a midline shift <5 mm and a hematoma width <10 mm, conservative management with ICP monitoring could be appropriate. The factors to be considered when a nonsurgical decision is taken are: GCS score; hematoma width and volume; midline shift; basal cistern collapse; time elapsed since trauma and CT scan; IPC monitoring, pupillary and motor responses; use of opioids, sedatives and muscle relaxants; coagulation disorders and urgent availability of CT and neurosurgical team services.
35.3.6 Prognosis
Mortality related to subdural hematoma is between 42 and 90% according to different series. Younger patients (36-50 years) have better outcomes. The mortality rate in patients lucid at surgery is 6-10% but increases 4-6 fold in comatose patients. The mortality rate is twice as high when pupillary abnormalities are present as when not (75 vs. 35%). Timing is also an important factor for prognosis; Seelig reported 40% mortality for patients in coma operated within the first 4 hours versus 90% mortality for those operated 4 hours after trauma. There is no general consensus on whether hematoma size is a predictor of poor prognosis; however, its association with other intraparenchymal lesions seems to be related to the prognosis. Jamieson and Yelland compared the mortality rate in 3 patient subgroups: subdural hematoma without associated lesions (22%); subdural hematoma with increased ICP and temporal lobe contusions (>50%); and subdural hematoma with contusions (30%). Increased ICP during the pre- and postoperative period predicted poor prognosis.
35.4 Intra-axial Lesions
Intra-axial lesions refer to those located inside the brain. They are caused secondary to a direct blow to the skull area over the brain, thus injuring the small calibre vasculature, and are generally seen over the cortical and subcortical areas. They may evolve and grow in size over time.
35.5 Contusion and Cerebral Hemorrhage
Contusions are areas of brain damage with a variable degree of edema, ischemia, necrosis and bleeding. They may increase in volume with time, and coalesce to form an intracerebral hemorrhage. The edema that may be present from the beginning may also increase and generate mass effect. Intracerebral hemorrhage is a homogeneous collection of blood with well-differentiated margins and considerable diameter. Differentiating it from contusion is not usually easy.
Contusions are more frequent (89% of cases on autopsy) and are more often demonstrated on CT than intracerebral hemorrhage (20-40% vs. 15-20%, respectively). They are usually located on the frontal and temporal lobes. They tend more frequently to be multiple, whereas intracerebral hemorrhage is multiple in only 20% of cases.
Hypodense areas around contusions and intracerebral hemorrhage are related to low cerebral blood flow. Cytotoxic substances are released from these contused areas into the surrounding tissue and blood stream. The formation of free radicals, excitatory amino acids and the altered homeostasis of Ca and K in these areas further generate tissue injury. Some authors have argued that evacuation of these lesions may help to preserve more vulnerable cerebral areas.
35.5.1 Clinic Manifestations
Although contusions and intracerebal hemorrhage do not have a typical clinical presentation, more than 50% of patients present with a GCS score <8. Lesions located on eloquent areas will cause neurological deficits. Alteration of the level of consciousness may be due to big volumes and consequent ICP elevation or the association of diffuse bilateral lesions compromising the brainstem. The lesion may grow with time. Importantly, the definite volume will not necessarily show on the first CT scan, which is why CT scanning should be repeated every 8-12 hours, especially during first hours after trauma. Another dynamic component is edema which may evolve over the ensuing 7-10 days and cause delayed elevated ICP requiring prolonged ICP monitoring. A CT scan should be obtained 4-6 days after the trauma to determine evolution of the edema. Contusions and intracerebral hemorrhage on the temporal lobe may cause uncal herniation without increasing ICP. Surgical evacuation of lesions in this area with evidence of basal cistern collapse is advised to prevent this complication.
35.5.2 Diagnosis
The diagnosis is based on CT findings which show a pattern of multiple hyperdense spots <1 cm diameter surrounded by a circular hypodense area compatible with peripheral edema. Intracerebral hemorrhage is seen as hyperdense generally homogeneous lesions with a peripheral area of edema or ischemia that is better defined the day after the trauma (Figure 35.7).
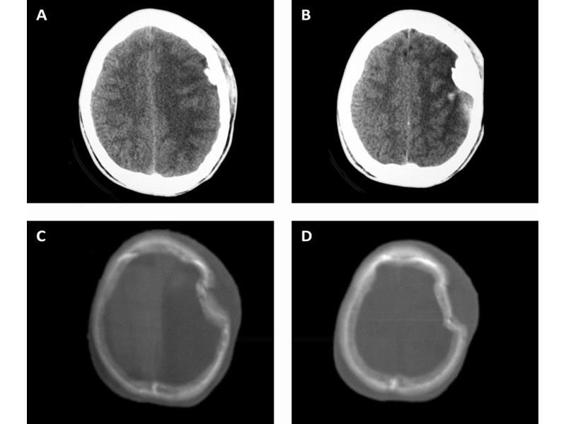
Figure 35.7. CT scan showing a left depressed skull fracture with an area of contusion and bone fragments over the brain (A). CT scan with bone window view showing the depressed skull fracture with a huge cephalo-hematoma (B).
35.5.3 Surgical Treatment
Contusions or intracerebral hemorrhage with mass effect and progressive neurological deterioration must be evacuated. Contusions over silent areas should be widely evacuated trying to resect all devitalized tissue, whereas the resection should be more modest in those located in eloquent areas. Small contusions not responsible for neurological deterioration, such as those located on the basal ganglia or white matter, should not be resected. Table 35.3 lists the recommendations for the management of these lesions [3].
Indications for surgery | Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|



