Fig. 21.1
An unusual presentation of unilateral brain, skull, and cutis abscess due to neurobrucellosis observed in a 64-year-old man, a country worker who gave no importance to the skin lesion, with a very low educational level and a history of fever, headache, vomiting, and seizures. When he arrived to the hospital, his abscess was initially misdiagnosed because he was comatose with a GCS score of 9 (a). During the operation the skull defect was enlarged with a wide craniotomy and curettage of the intracerebral portion of the abscess was performed, with a pathological diagnosis of brucellosis (b)
The established guiding indications for surgical management are confirmation of microbiological diagnosis; antibiotic therapy modification from obtained pus; unresponsiveness to medical management; significant clinical deterioration; a walled off abscess larger than 3 cm in diameter; a capsular staged abscess larger than 2 cm in diameter; deep located capsular staged abscess larger than 3 cm in diameter; cerebellar, subdural, epidural, brain-stem, or paraventricular abscess; and finally the presence of multiple lesions [4, 89, 93, 94, 110].
21.2.3 Operative Procedures
Surgical therapy provides samples for accurate diagnosis, reduces the mass of the abscess, improves the efficacy of the drug used for treatment, and in some conditions allows intrathecal, intraventricular, and intracavitary administration of the antibiotic agent [53]. Currently, the principal methods for surgical management involve freehand or endoscope-assisted aspiration of the pus with/without stereotactic CT guidance, drainage by craniotomy or craniectomy with/without intraoperative ultrasound (US) guidance, or excision of the abscess. The choice of procedure is a matter of debate, but the preference and the ability of the surgeon, as well as the ability of the patient to tolerate each procedure, should be taken into account [6, 88]. Craniotomy was advocated in the pre-CT era but is now rarely practiced as the first line of treatment [88]. Aspiration, repeated as necessary or combined with drainage, has widely replaced attempts at complete excision. Several reports have still advocated excision as the procedure of choice, because it is often followed by a lower incidence of recurrence and shorter hospitalization [88].
21.2.3.1 Aspiration
In most cases, aspiration of the purulent material is sufficient to initiate healing of the abscess [4]. Aspiration can be used in the cerebritis stage where biopsy gives positive culture and can discriminate abscess from other brain lesions (tuberculoma, metastasis, glioma, resolving hematoma) [106, 109]. Weekly or biweekly CT scans to monitor the size of the abscess are mandatory following aspiration or drainage in addition to parenteral antibiotic therapy for 6–8 weeks [82]. More than one aspiration may be needed for cure [109].
21.2.3.2 CT-Guided Stereotactic Procedure
Deep-seated abscess should be drained by a CT-guided stereotactic procedure [88]. Real-time US, particularly in infants with open fontanels, provides precise localization [110]. Multiloculated abscesses have been treated with stereotactic aspiration of all loculi in single or staged aspiration [109]. A question has been raised as to whether aspiration combined with immediate washout, with or without antibiotics, and drainage for several days could lead to even better results, with this point seeming to be more important for the treatment of subdural empyemas [102]. Sometimes though, the penetration of a thick abscess wall with the blunt-tipped stereotactic probes can be difficult, and one may fail to enter the abscess [83]. Impedance monitoring can avoid the false-negative result [101]. The ability to monitor the progress of the aspiration in real time and to detect intraoperative complications makes the use of the open magnetic resonance imaging (MRI) within the operating room attractive, although its availability is still extremely limited [64, 102]. Stranjalis [121] applied a stereotactic aspiration in a patient with a cerebral abscess due to Brucella. In general, aspiration is a rapid and safe procedure especially with the use of stereotactic US or CT-guided techniques, which can be done even under local anesthesia, on bedside, in seriously ill high-risk patients [110]. Often, the initial approach is to drain the abscess through a twist-drill craniostomy [88].
21.2.3.3 Burr Hole Drainage
If the pus is thick or inadequate drainage of abscess is suspected, the next procedure would be therapeutic burr hole evacuation with or without intraoperative radiography and neuronavigation [88]. The residual pus can be evacuated if the patient does not exhibit significant improvement, or serial CT reveals moderate to large residue [88].
21.2.3.4 Neuroendoscopic Stereotactic Evacuation
Neuroendoscopic stereotactic evacuation with rigid or flexible scope has shown encouraging results and additional advantages of more complete drainage and lavage [42, 55, 59, 73]. Deep-seated (thalamic, basal ganglia, brain stem) and hemispheric eloquent area abscess can be managed by frame-based stereotactic or neuronavigation-guided aspiration [83, 88].
21.2.3.5 Drainage by Craniotomy, Craniectomy, or Excision
Drainage by craniotomy, craniectomy, or excision is used more often in superficial abscesses and those found in the posterior cranial fossa (Fig. 21.1b) [6]. Surgical excision becomes mandatory if the pus is thick, if a peripherally placed abscess fails to respond to aspiration, and in multiloculated abscesses [4]. However, excision is inappropriate in the cerebritis stage; small, deep-seated abscesses in eloquent areas; and multiple abscesses [4, 110].
Open craniotomy for excision of brain abscess allows complete removal of purulent material and the surrounding abscess capsule, providing definitive treatment, like in a reported case of refractory epilepsy caused by a chronic abscess in brucellosis [22, 44]. The capsule often has anchor extensions into the surrounding white matter, and the surgical procedure may cause unplanned extensive damage to the adjacent viable cerebral tissue [54]. The ideal excision of the abscess in one piece, necessary to prevent the spillage of pus in the operative field and the reinfection of the traumatized brain tissue, contradicts the general neurosurgical tenet that removal of debulked lesions causes significantly less parenchymal and vascular brain damage [102].
21.2.3.6 Balloon Catheter-Assisted Excision
A balloon catheter-assisted excision averts spillage and allows the decompressed abscess to be pulled gently toward the surface while the cleavage plane from the brain is developed step by step [102]. In cases where complete excision is not feasible (wall of the abscess appeared too thin on US) during operation, open aspiration via craniotomy allows thorough irrigation of the abscess cavity and verification of complete evacuation of abscess contents using US [44].
21.2.3.7 Serial or Staged Stereotactic Aspiration
Serial or staged stereotactic aspiration can be performed in abscesses greater than 3 cm in diameter, deeply located in the brain stem or close to the ventricular wall, recollecting or enlarging, and failing to decrease or resolve [109, 110]. When a peripherally placed abscess fails to respond to aspiration, consideration should be given to craniotomy and excision [4]. In multiple abscesses antibiotics are continued for 3 months [110]. Calik et al. [20] described a case of multiple brain abscesses and hydrocephalus in a 4-month-old infant, due to Brucella melitensis after isolation from cerebrospinal fluid (CSF), which was treated only with ventriculoperitoneal shunt and long-term antibiotic administration, but no abscess surgical drainage.
21.2.3.8 Craniotomy
A craniotomy should be performed for an epidural abscess, with a large osteoplastic craniotomy to be preferred in comparison with a free flap because of its better vascularity and its increased resistance to infection [102].
21.2.3.9 Ventriculostomy
Urgent evacuation of abscess is required for subdural empyema and cerebellar abscess, and a ventriculostomy is indicated for significantly IICP [88]. Tabatabai et al. [122] drained a delayed postsurgical subdural empyema in brucellosis via a burr hole-applied technique. Shoshan et al. [111] reported a surgical drained chronic subdural empyema during craniotomy in an 8-year-old girl, with the characteristic MRI appearance of diploic widening together with the thick capsule and meningeal-pial enhancement. Subdural empyema in infants may reoccur after repeated drainage and can be successfully resolved by craniectomy and juxtaposition of the temporal muscle [26]. Cerebellar abscess due to brucellosis has been reported [11]. Cerebellar or brain stem abscess is often an indication for posterior fossa craniotomy [4].
21.2.3.10 Cerebrospinal Fluid Diversion
Associated supra- or infratentorial abscess or empyema may be present with cerebellar abscess, but the latter one needs to be regarded differently from supratentorial abscess because of its ability to cause sudden total occlusion of CSF pathways early in the course of disease [88, 110]. Immediate CSF diversion with an external ventricular drain is mandatory in presence of overt or incipient hydrocephalus [95]. Persistent hydrocephalus is treated with a shunting procedure [88]. Presence of periventricular lucency is an absolute indication for immediate ventricular drainage regardless of level of consciousness [88].
Regardless of the suggestion that cerebellar abscess should be managed by primary excision, in recent years burr hole aspiration has emerged as a satisfactory method [19]. Drainage via a twist-drill craniostomy is as effective as burr hole drainage but is best avoided except in life-threatening situations or as a salvage procedure [88]. Cerebellar abscess should be completely excised through a suboccipital craniectomy or a craniotomy [88]. Solaroglu et al. [114] reported a case of surgical cyst aspiration with wide capsule resection via a bilateral suboccipital craniectomy and C1 laminectomy after an ineffective operative spinal needle aspiration, in a patient with one solitary extra-axial posterior fossa abscess due to brucellosis. They were unable to remove completely the capsule of the abscess as the anterior part was firmly adhered to the brain stem [114]. Gündeş et al. [50] evacuated a left cerebellar hemisphere brucellar abscess via an occipital (supratentorial) craniotomy. In cases of intraventricular rupture of brain abscesses, in addition to a combination of intrathecal and intravenous antimicrobial treatment, rapid evacuation and debridement of the abscess cavity via urgent craniotomy, lavage of the ventricles, intraventricular drainage, and intraventricular administration of antibiotics are recommended [72].
21.2.4 Complications and Outcome
The two most feared complications of cerebral abscess are herniation secondary to mass and abscess rupture into the ventricle and/or subarachnoid space [46]. In some rare cases, uncoagulated old hematoma-like fluid such as chronic subdural hematoma fills the drained or excised cavity, as a result of the significant revascularization with inflammatory cells in the remaining abscess capsule or cavity [102]. After aspiration and drainage of brain abscess, delayed minor bleeding from the capsule can be detected by MRI [131]. Late recurrent abscess is more common after aspiration alone than after excision [46].
About 30–50 % of survivors are found to have neurological sequelae, which fall into three major categories: focal neurological deficits, cognitive and learning impairment, and seizures [46, 110]. Epilepsy may occur during long-term follow-up; therefore, all patients with subdural empyema should be placed on anticonvulsants for at least several months after surgery [102].
Although the use of steroid treatment in brain abscess is controversial, corticosteroids may be beneficial in patients with IICP, significant cerebral edema with mass effect, compromised mental or neurological status, and potentially life-threatening complications such as impending cerebral herniation, despite maximal surgical treatment [39, 57]. Some feel that steroid therapy can reduce antibiotic penetration into the abscess or increase the risk of intraventricular rupture [44].
At present, major centers report a mortality rate of less than 10 % [71]. Many poor prognostic indicators have been described, which include neonates, infants, and elderly patients; delayed diagnosis and institution of treatment; rapidly progressing disease; sensory deterioration at presentation; coma; multiple and deep-seated lesions; intraventricular rupture and posterior fossa location; associated meningitis, ependymitis, or empyema; large size; and presence of hydrocephalus [4, 110]. Early aspiration, together with the absence of initial seizures, sterile CSF, and normal ventricles on CT scan, appeared to be a factor leading to a better prognosis in terms of epilepsy and mental sequelae [6].
Primary excision of brain abscess carries the risk of serious damage to the surrounding brain tissue with increased potential for neurological sequelae and epilepsy [4, 93]. Today, aspiration of brain abscess has become the preferred method of surgical drainage, with main disadvantages of the repeat procedures (because incomplete evacuation of thick purulent material), the hemorrhage into the abscess capsule and the possibility of iatrogenic puncture of ventricle, and subarachnoid or subdural leakage of pus leading to meningitis, or empyema, or ventriculitis [41, 54, 94].
Stereotactic aspiration avoids the so-called leukotomy effect that can occur with a freehand aspiration technique [83]. The incidence of residual neural deficits, such as hemiparesis, and cognitive and learning deficits in children is less with aspiration than excision [110]. Excision has been better than aspiration in regard to postoperative duration of antibiotics use, earlier improvement in neurological function and radiological clearance, rate of reoperative surgery, length of hospital stay, and overall cost of treatment [4, 87, 105, 106, 123].
21.3 Surgical Therapy of Neurobrucellosis with Cerebrovascular Involvement
Meningovascular complications of neurobrucellosis are reported in the medical literature, and some of them may lead to clinical presentations that necessitate urgent intervention [49]. Subarachnoid hemorrhage (SAH), subdural hematomas, mycotic aneurysms with or without rupture, ischemic attacks, and venous thrombosis are vascular involvement of neurobrucellosis [1, 3, 14, 15, 49].
Altekin et al. [3] described a case of intraparenchymal hematoma caused by rupture of a mycotic aneurysm of the right posterior cerebral artery in a patient with aortic valve endocarditis caused by Brucella melitensis. Because of high complication risk and low success rate of endovascular treatment without infection control and the patient’s neurological stability, intervention for the aneurysm was planned after infection control and aortic valve surgery [3]. Although mycotic aneurysms are fragile, hemorrhage is an uncommon event, supporting more the conservative approach with prolonged courses of antibiotics and serial follow-up angiography to detect the dynamic or stable nature of aneurysm [91].
In a dynamic aneurysm, the aim of aggressive surgical therapy is to eliminate the mycotic aneurysm from circulation, prevent the ischemic events from arterial circulation, and evacuate any associated hematoma [28, 91]. Endovascular treatment of mycotic aneurysm due to neurobrucellosis can be achieved safely after the infection and vascular inflammation are taken under control [3]. Delayed surgery may reduce the risk of perioperative rupture and allow the aneurysm to mature from a friable acute lesion to a more fibrotic chronic lesion [28]. Erdoğan et al. [38] described a case of ruptured aneurysm of the distal anterior cerebral artery (ACA) associated with brucellosis, who presented with the symptoms of SAH. A right frontal interhemispheric approach was used, and a tiny aneurysm that had not been detected on angiography was found arising from the left distal ACA [39]. The ruptured fusiform aneurysm at the right callosomarginal artery was successfully clipped, and then the silent aneurysm was covered with muscle [39].
21.3.1 Endovascular and Surgical Techniques
21.3.1.1 Intracranial Stents
Intracranial stents provide the unique ability to simultaneously preserve parent vessel integrity while obliterating the aneurysmal sac, but their use for the treatment of mycotic intracranial aneurysms has only been reported in a few instances [34].
Ay presented a patient with brucellosis and a frontoparietotemporal infarct in the right cortical area, supplied by the middle cerebral artery (MCA) and a prominent stenotic segment distal to the right MCA. Reperfusion was achieved with a stent placed into the right MCA [10]. Although evidence regarding the efficacy of endovascular treatment in acute stroke has been equivocal, the recent publication of a large multicenter randomized controlled trial indicates benefit of intra-arterial stent retriever reperfusion in patients selected by appropriate imaging and treated early by experienced operators [8].
21.3.1.2 Endovascular Embolization or Trapping
Selective endovascular embolization or trapping with soft and ultrasoft detachable coils is an effective technique that should be considered for management of dynamic unruptured mycotic aneurysms, whereas conventional surgical intervention should be restricted to ruptured mycotic aneurysms with associated intracerebral hematoma and IICP [91]. Amiri et al. [5] reported a case of a small intracerebral and intraventricular hemorrhage accompanied with mild hydrocephalus occurring in a patient with three Brucella-related mycotic aneurysms treated with delayed surgery, resection, and excision after a left posterior temporoparietal craniotomy.
21.3.1.3 Sinus Thrombectomy, Bypass, Thrombolysis, and Clot Disruption
Zaidan and Al Tahan [134] described a case of complete sagittal sinus thrombosis in a patient with meningitis due to Brucella melitensis and a pseudotumor-like picture. The value of neurosurgical intervention in dural sinus thrombosis remains unproved [62]. Decompressive craniotomy or craniectomy has been used in patients with coma and significantly IICP, although benefit is unproved and outcomes are commonly poor [27, 90].
Surgical revascularization procedure such as sinus thrombectomy and bypass has been reported, when peripherally infused anticoagulant or thrombolytic agents fail [112]. Also, local thrombolysis via a frontal burr hole has been described [107]. Endovascular local intrasinus thrombolysis is an increasingly applied intervention, but this modality carries an increased risk of hemorrhage [27]. A reasonable indication for endovascular therapy would seem to include thrombus propagation and clinical deterioration despite adequate anticoagulation and reduction in consciousness followed by seizures and hemiparesis [84]. The endovascular approach allows local sinus thrombolysis directly targeted into the thrombus and for mechanical disruption or aspiration of thrombus, both of which contribute to recanalization [84]. The most widely applied endovascular technique in combination with local thrombolysis is clot disruption, performed with angioplasty balloons, microwires, snares, or microcatheters [16, 24, 100, 115, 119]. Revascularization of the occluded sinuses by stent placement was also described [40, 58].
Several recent studies report on the successful use of the Angiojet rheolytic endovascular device, that works by creating a vacuum to fragment and aspirate the thrombus [27, 82, 136]. The use of the MERCI clot retrieval device was also applied for clot disruption in combination with thrombolysis [96]. The penumbra thrombectomy system is an alternative aspiration-debulking device [68]. Due to the relative rarity of the disease, more studies are required to establish the full role of invasive endovascular therapy in this patient population [68, 84].
21.4 Surgical Therapy of Neurobrucellosis with Intraparenchymal Cerebral Lesions
Intraparenchymal mass lesions mimicking a cerebral tumor, as a B. melitensis infection-associated syndrome, have been documented radiographically [37, 77, 80, 113]. In these cases, MRI revealed an enhanced lesion with vasogenic edema associated or not with midline shift and mass effect, interpreted as a cerebral tumor [37, 80, 113]. The patients were misdiagnosed as a brain tumor and underwent a surgical biopsy, with/without total excision of the lesions, or partial lobectomy [37, 39, 113]. The diagnosis of granulomatous or non-granulomatous encephalitis was made after the mass biopsy, but histopathologic studies did not reveal the organism in tissue sections [37, 39, 113]. Only in one case the definitive diagnosis of neurobrucellosis was made via bacterial isolation of Brucella in postoperative brain tissue specimen culture [113]. Residual areas of brain atrophy and focal neurological deficits are possible long-term complications [37, 39, 113].
Neurobrucellosis must be considered in the differential diagnosis of cerebral tumors, particularly in endemic areas, especially if the patient has a history of previous brucellosis. Patients having these lesions, with either granulomatous or non-granulomatous encephalitis, should be studied for brucellosis with blood and CSF cultures and serological tests [37].
21.5 Surgical Therapy of Spinal Neurobrucellosis
The intimate anatomical association of the spinal cord and the spinal nerve routes with the vertebral column renders spinal neurobrucellosis in a direct relationship with the various forms of vertebral brucellosis. Spinal involvement of brucellosis was first described in 1932 [92]. Spondylitis and spondylodiscitis are the most common forms of osteoarticular brucellosis with a reported incidence from 10 % up to 60 % in different studies [12, 30, 31, 36, 63, 98, 126, 127]. Other spinal clinical types are arthritis, osteomyelitis, and paravertebral and epidural abscess [33].
The most frequent area of involvement in spinal brucellosis is the lumbosacral region, followed by the thoracic and cervical segments [30, 31, 116, 126]. In rare occasions the disease may affect simultaneously different vertebral segments [137]. Spondylodiscitis is a very serious form of Brucella osteoarticular involvement [128]. Brucella reaches the spinal central nervous divisions via either hematogenous route or by direct extension from spondylitis [92]. It may spread up to the cervical spine via the CSF route [92]. Epidural abscess or granuloma, radiculoneuritis, myelitis, and demyelinating neuropathy are the most critical causes of morbidity [7, 17, 30, 128].
Significant progress has been made on the guidelines of conservative and surgical therapy, thanks to the evolution of accurate diagnostic tools such as MRI and fine operative techniques and the invention of sophisticated operative spine stabilization devices. Though spinal neurobrucellosis is not as fatal as in other conditions such as brain abscess, it is a serious factor of morbidity, chronic disability, and medical care cost.
Before outlining the indications of surgical treatment of spinal neurobrucellosis, there are some points regarding the disease which affect the timing and the outcome of surgery, and they must be kept in mind. The point of surgical intervention in spinal neurobrucellosis has been under debate [128]. Brucellosis has a slowly progressive course with unspecific symptoms [12]. Spinal neurobrucellosis usually responds well to combined antibiotic therapy even in cases with serious and progressive neurological symptoms, such as spastic paraparesis, and sphincter abnormalities in bowel and bladder functions [45, 60]. The extent of conservative treatment usually may vary from 8 weeks to 2 years [130]. This duration combined with the efficacy of modern conservative treatment is a factor which renders the timing of surgical intervention in question [12, 117].
A serious factor that has to be considered is that the therapeutic decisions are based on evidenced data which originate from a relatively small number of cases in the relevant literature. For example, 52 % of publications on spinal brucellosis out of a total number of 64 articles regarding 452 cases from 34 secondary or tertiary referral centers in Turkey were merely case reports [128]. It should also be noted that treatment recommendations for all types of bacteria causing spinal epidural abscess (which is one of the most aggressive complications of brucellar spondylodiscitis) are based on retrospective studies, case series, and expert opinions [108].
Laboratory information (55 % of blood cultures and 30–50 % of cultural tests of specimens from drained abscesses) may not be diagnostic for a long period of time [13, 48]. In many occasions there is a differential diagnostic dilemma from vertebral or intervertebral disk herniation (IVD), malignant tumors, and other infectious diseases such as spinal tuberculosis [13, 48, 133]. History of raw dairy product consumption, occupation dealing with livestock, and the geographical distribution of brucellosis are often the critical factors that guide the diagnostic investigation [13, 48, 128, 132]. All these factors set hurdles on the final decision of the surgical team that confronts with the neurobrucellosis patient in terms of diagnosis and choice of treatment.
MRI is the gold standard diagnostic method for the accurate assessment of the anatomy and topography of the lesions in spinal neurobrucellosis [128]. The characteristic radiological features of each lesion have been coded, whereas experience is increasing [128].
Some authors suggest immediate and aggressive surgical intervention, especially in epidural spine abscesses, in cases with severe neurological deficits (spinal instability, cord compression, radiculopathy, cauda equina syndrome, severe muscle weakness, progressive crumple), fearing a significant risk of sudden neurological deterioration and vertebral collapse during the period of treatment with antibiotics [2, 30, 31, 117, 135]. The authors of this mentality also support that prompt surgical therapy can prevent and decrease severity, that the combination of medical and surgical treatment is necessary to alleviate the disease and shorten the time of therapy, and that they leave conservative treatment with close observation in selected cases, particularly the elderly [31, 51, 133].
There are more roles of surgery in the confrontation with spinal neurobrucellosis. One of them is the palliation and reduction of the mass effect on the neighboring neural elements; as the lesions from Brucella microorganisms are slow growing, encapsulated, elastic in nature, moderately vascular, and firmly adhered to neural structures, the dissection is challenging, with a serious possibility of severe complication of CSF perioperative leakage [31]. There are also authors who suggested percutaneous drainage or aspiration of epidural and paravertebral abscesses instead of surgery [124].
As shown in the algorithm, the location of surgical intervention in the multidisciplinary and time-consuming effort of treatment is actually in the last raw of the relevant algorithm [128]. Nevertheless, surgeons are familiar with this position of their role as it is obvious from the last pages reserved for the surgical therapy in any major medical textbook (Fig. 21.2).
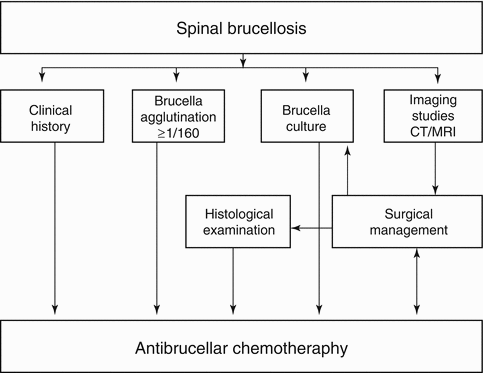





Fig. 21.2
Algorithm depicting the diagnosis, imaging, and treatment management pathway for spinal brucellosis (From Turgut et al. [128])
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







