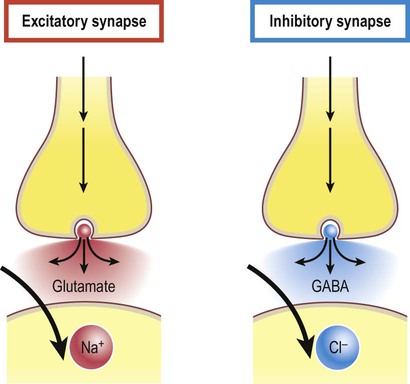
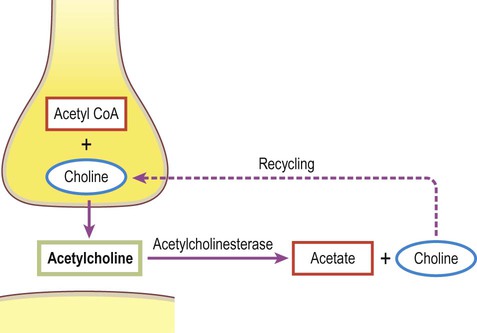
A gap junction is composed of around 100 intercellular channels called connexons that are inserted into the plasma membranes of adjacent cells (Fig. 7.1). Each connexon is composed of a hexagonal array of proteins called connexins, surrounding an aqueous channel that is 2 nm wide. The pores in adjacent cell membranes are aligned to form a ‘tunnel’ between the two cells. These can be opened or closed by a conformational change in the constituent proteins, regulated by phosphorylation state. Gap junctions represent a low-resistance pathway that allows charged particles and small molecules to flow freely in either direction and couples the electrical activity of adjoining cells. Groups of cells linked by gap junctions form an electrical syncytium which can generate large, synchronized discharges. This happens in certain brain stem nuclei that control breathing and may contribute to the generation of abnormally synchronized discharges in some forms of epilepsy (Ch. 11). Most central nervous system synapses are chemical. The general structure and arrangement is similar to that of the neuromuscular junction (see Ch. 4) and a great deal of information about central synapses has been derived from experiments at the point of contact between nerve and muscle. Peptide transmitters are synthesized in the cell body and pre-loaded into vesicles which are delivered to the axon terminal by fast axonal transport (see Ch. 5). Other transmitters are synthesized within the axon terminal and are loaded into synaptic vesicles by transport pumps in the vesicle membrane. Some are synthesized within the vesicle itself. Synapses can be excitatory or inhibitory. If the presynaptic neuron releases an excitatory transmitter (most commonly glutamate) then the membrane of the postsynaptic cell will be depolarized. This is referred to as an excitatory post-synaptic potential (EPSP) and is typically associated with an inward sodium current. If the presynaptic neuron releases an inhibitory neurotransmitter (the most common of which is gamma-aminobutyric acid or GABA) then the postsynaptic membrane will be hyperpolarized (inhibited). This is an inhibitory postsynaptic potential (IPSP) and is often associated with an inward chloride current (or an outward potassium current). EPSPs and IPSPs are graded potentials of a few millivolts that only influence the local membrane and then rapidly decay (see Ch. 6). Classical, small molecule neurotransmitters are stored in clear-cored vesicles that are approximately 50 nm in diameter. The first to be identified was acetylcholine (ACh) and it remains the best understood. Acetylcholine is found at the neuromuscular junction and throughout the autonomic nervous system. It differs from other types of neurotransmitter and has a distinct chemical structure, biosynthesis and mechanism of inactivation (Fig. 7.5). Acetylcholine is synthesized in axon terminals from two metabolites that are present in all cells: acetyl-coA and choline. This is achieved by the enzyme choline acetyltransferase (ChAT) which is only present in cholinergic neurons. The remaining small molecule neurotransmitters are divided into amino acids and biogenic amines.
Synaptic transmission
General principles
Electrical synapses
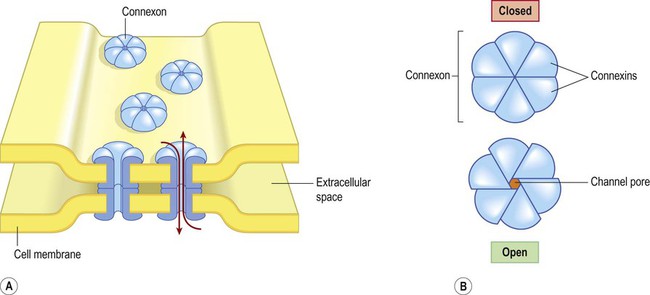
(A) Gap junctions are points of direct communication between the cytoplasm of adjacent cells, formed by protein complexes called connexons; (B) Each connexon is composed of six connexin subunits, surrounding a central pore.
Chemical synapses
General structure (Fig. 7.2)
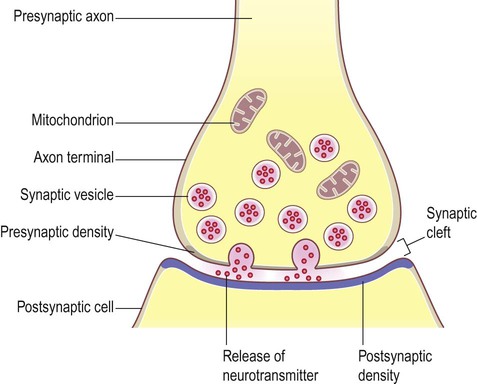
The arrangement is similar to that of the neuromuscular junction between nerve fibres and skeletal muscle.
Excitatory and inhibitory synapses (Fig. 7.3)
Release of neurotransmitter (Fig. 7.4)
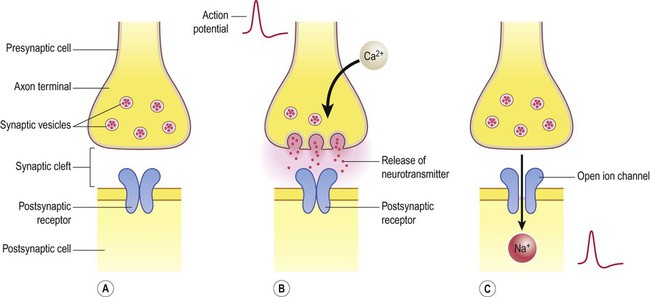
(A) Neurotransmitter molecules are stored in the presynaptic element within membrane-bound synaptic vesicles; (B) Arrival of an action potential at the axon terminal triggers calcium influx via voltage-gated calcium channels which causes the vesicles to fuse with the presynaptic membrane and discharge their contents into the synaptic cleft; (C) The transmitter diffuses across the synaptic cleft and interacts with receptors on the postsynaptic membrane, leading to ion fluxes that will either inhibit or (as in this case) excite the postsynaptic neuron.
Neurotransmitters
Classical neurotransmitters
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Synaptic transmission
Only gold members can continue reading. Log In or Register to continue