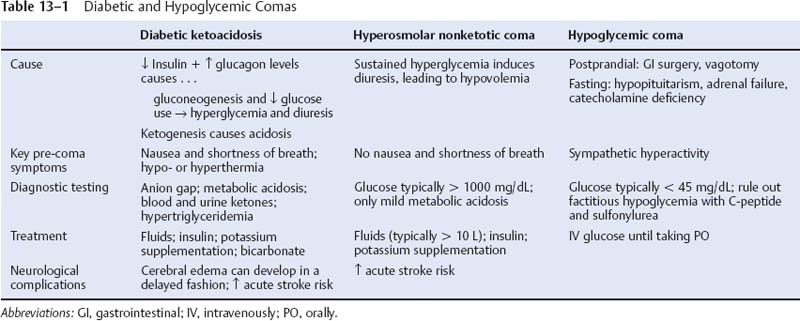
13 Note: Significant diseases are indicated in bold and syndromes in italics. 1. Subtypes a. type I/insulin-dependent DM: caused by autoimmune destruction of islet cells of the pancreas leading to insulin deficiency i. common antibodies include those against insulin and glutamate decarboxylase (Box 13.1) ii. typical patient is < 45 years of age, thin, and prone to ketosis; frequently associated with other autoimmune diseases b. type 2/noninsulin-dependent DM: caused by peripheral tissue insulin resistance; not associated with autoimmune diseases i. typical patient is > 30 years of age, obese, and ketosis-resistant c. secondary diabetes: from glucocorticoid administration, Cushing’s syndrome, acromegaly, or pheochromocytoma 2. Diabetic neuropathies: identification of diabetic neuropathy is complicated by the association of DM with other neuromuscular diseases (ataxia telangiectasia, Friedreich’s ataxia, myotonic dystrophy, amyloidosis, porphyria, glycogen storage diseases, mitochondrial myopathies, and stiff man’s syndrome) a. subtypes i. symmetric diabetic neuropathies (1) diabetic chronic sensorimotor neuropathy—most common form of DM neuropathy (30% of all DM neuropathy) (a) pathophysiology: glucose is converted to sorbitol by aldose reductase and sorbitol dehydrogenase, and the accumulation of intracellular sorbitol causes cellular edema that injures primarily the vasculature and the Schwann cells (i) histology: microvascular thickening with hyalinization, and axonal degeneration of large and small fibers; complement deposition occurs in the microvasculature with inflammatory infiltrates (ii) risk factors include obesity, diastolic hypertension, dyslipidemia, inadequate glucose control, and other end-organ damage (albuminuria, retinopathy) (b) symptoms (i) distal pain and paresthesias (ii) distal sensory loss leading to foot ulcerations and neuropathic osteoarthropathy (Charcot’s joints) (iii) mild weakness; areflexia (iv) may have some dysautonomia (2) diabetic autonomic neuropathy (a) pathophysiology: degeneration of CN X and other autonomic nerves (ii) risk factors include duration of diabetes, adequacy of glucose control, diabetic end organ damage (albuminuria, retinopathy), age, obesity, and dyslipidemia (b) symptoms (i) cardiovascular: high resting heart rate, orthostatic hypotension (ii) genitourinary: erectile dysfunction with preserved ejaculation and orgasm; urinary retention (rare) (iii) gastrointestinal: constipation; gastroparesis, nocturnal diarrhea (rare) (3) diabetic acute painful neuropathy (a) risk factors include inadequate glucose control, rapid weight loss, and the recent initiation of insulin therapy (b) symptoms: distal pain with mild sensory loss ii. asymmetric and focal diabetic neuropathies (1) proximal diabetic plexopathy/diabetic amyotrophy/Bruns-Garland syndrome (a) pathophysiology: immunoglobulin M (IgM) and complement deposition in endoneurium leading to macrophage infiltration of the microvasculature of the lumbosacral plexus and/or upper lumbar roots (i) more common in type II DM (ii) risk factors include inadequate glucose control and rapid weight loss (b) symptoms (i) pain in the hip, buttocks, or thigh (ii) weakness in the proximal lower extremity muscles that usually involves muscles not innervated by the femoral nerve, therefore it is not equivalent to a diabetic femoral neuropathy (iii) minimal sensory loss (c) diagnostic testing: EMG demonstrates multifocal denervation in the paraspinous muscles indicating the involvement of the nerve roots (2) diabetic mononeuropathy (a) epidemiology: tends to occur in older patients (b) symptoms: neuropathies are generally painful; commonly involves (i) CN III (pupil-sparing) (ii) CN VII (iii) peripheral nerves (particularly those of the lumbosacral plexus) that often mimic an entrapment neuropathy (e.g., median nerve at the carpel tunnel, ulnar nerve at elbow, posterior tibial nerve at tarsal tunnel) (Box 13.2) (iv) peripheral nerves of the trunk, wherein the weakened abdominal muscles allow for hernia formation b. treatment: intensive control of blood glucose, which reduces neuropathy risk by 60% i. target HgA1c (a glycosylated hemoglobin) to < 7%, although there is no threshold for developing neuropathy 3. Diabetic myopathy (≠ diabetic amyotrophy) a. pathophysiology: unknown, but likely due to a microangiopathy in the muscle i. risk factors include inadequate glucose control, other end organ damage (retinopathy, nephropathy, neuropathy), and hypertension ii. epidemiology: more common in women and type I diabetics b. symptoms: acute pain, tenderness, and swelling of the muscle, which is exacerbated by movement; usually located in the anterior or medial thigh > calf c. diagnostic testing i. normal or elevated CK ii. elevated erythrocyte sedimentation rate (ESR), usually > 100 iii. plain films can demonstrate vascular mineralization iv. MRI: demonstrates edema in muscle and perifascial space with fluid collection in tissue planes; minimal contrast enhancement v. muscle biopsy shows necrotic muscle fibers, hyaline thickening of the arterioles, and perivascular inflammation d. treatment: rest; analgesics; gradual mobilization e. prognosis: spontaneous resolution over weeks to months; recurs in 60%, but not necessarily in the same muscle group 4. Diabetic and hypoglycemic comas (Table 13–1) 1. Pathophysiology a. definitions: normal blood pressure is defined as systolic < 130 mmHg and diastolic < 85 mmHg; mild hypertension begins at systolic > 140 mmHg and diastolic > 90 mmHg i. hypertensive urgency: hypertension without significant symptoms or laboratory abnormalities ii. hypertensive emergencies: stage III hypertension that involves end-organ failure b. causes i. primary/essential hypertension (95%) ii. secondary hypertension (1) renal parenchymal diseases > renovascular diseases (2) endocrine: primary aldosteronism, Cushing’s disease, pheochromocytoma, hypercalcemia during hyperparathyroidism, oral contraceptive use (3) aortic dissection or coarctation (4) iatrogenic: hypercarbia, hypoxia, hypoglycemia, pulmonary edema (5) neurological (rare): status epilepticus, elevated intracranial pressure (a) Cushing reflex—hypertension + bradycardia + respiratory irregularity, reflecting dysfunction of the medulla (i) development of the Cushing reflex is predominantly related to the size of an intracranial mass, not the rate of its expansion 2. Symptoms a. central nervous system i. headache, typically occipital and most severe in the morning ii. lightheadedness and syncope; vertigo, tinnitus; visual blurring iii. stroke iv. hypertensive encephalopathy (1)
Systemic Diseases Affecting the Nervous System
I. Diabetes Mellitus (DM)
II. Hypertension
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree