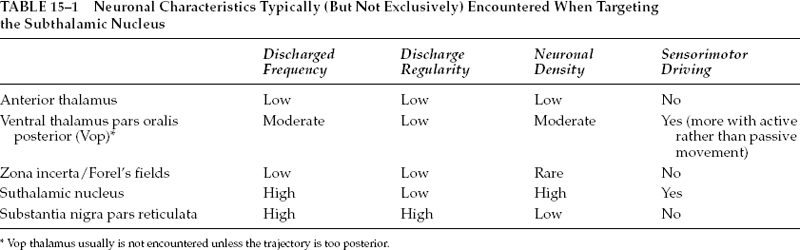
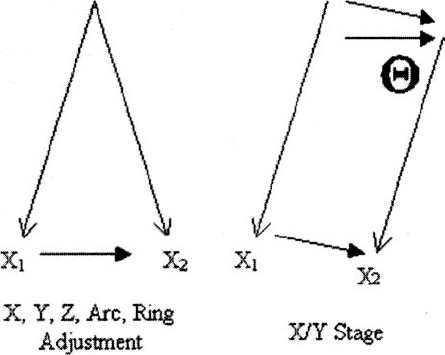
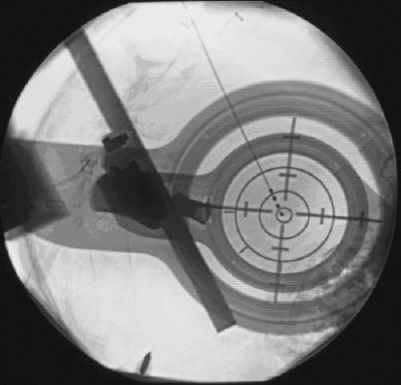
15 Much of the background for microelectrode-aided target localization has been provided in the preceding chapters of this volume, including the characteristic neuronal activities of the most commonly targeted regions of the brain, equipment choices and issues, operating room adaptations, and general principles of neurophysiology. Although this information is critical to successful target localization, it is not sufficient. It is equally as important to recognize the activity and response to microstimulation of neighboring structures, as this information may verify the appropriateness or inappropriateness of a given trajectory. Achieving optimal target localization requires a team with a collective understanding of stereotactic procedures, neurophysiology, including extracellular recording, and the regional anatomy. This information must be incorporated into a systematic approach to using intraoperative microelectrode recording in target localization. In the current chapter, we will provide a description of our own approach to intraoperative MER. A necessary note of caution is that the opinions described below are just that, opinions, and we recognize that other experts may have different opinions equally deserving of consideration. It is important to remember that MER methods have not been validated in a comprehensive, prospective, controlled, blinded fashion. Indeed, it may not be feasible to do so. The ultimate validation would be measured by clinical outcomes. Given the variability of clinical effects of surgery and the measures used to assess outcomes, larger sample sizes than are feasible or reasonable may be necessary. Despite the lack of precise validation by randomized clinical trials, physicians are compelled to provide surgery, particularly deep brain stimulation, because of the clear clinical efficacy1,2 and patients’ needs. The lack of randomized, placebo-controlled, blinded prospective studies does not relieve the physician of the necessity of considering other levels of evidence. Considerations in the use of MER include estimates of the technology’s impact not only on clinical efficacy but also on medical and financial costs and risks. It is important to distinguish a difference in approach or strategy for MER from a difference in equipment choices, and certainly the approach or strategy should drive the choice of equipment and not the other way around. Ultimately, either a remote-controlled hydraulic or a manual, turn-screw microdrive can pass an electrode forward and backward through the brain. However, each brings with it a set of difficulties and nuances. Similarly, a homemade collection of amplifiers and filters can be combined to create a respectable intraoperative monitoring equipment rack, or, alternatively, one may choose from any of several commercially available systems. Our own system has evolved from a handpicked, self-assembled collection of components from various manufacturers to include the use of a more self-contained, commercially available intraoperative recording system. Although there are advantages and disadvantages to each type of system, we have noticed little or no change in our actual approach as a result of this evolution. We use a single microelectrode approach in which the microelectrode is repositioned in any horizontal and sagittal planes to offer greater flexibility. The disadvantage of needing to study the entire trajectory using a single microelectrode is offset by the fact that, at least for targeting the subthalamic nucleus, fewer penetrations of the brain are needed. In our hands, this approach has enabled us to find the optimal trajectory with an average of 1.44 penetrations (unpublished data). It is rare to require more than two penetrations in a single subthalamic nucleus. Not only does such an approach save time, it also reduces risk; fewer electrode passes confined to a single pial penetration are less likely to transgress a deep sulcus inadvertently and less likely to encounter a random deep vessel. Clinical trials have shown increased risk of hemorrhage with increasing numbers of brain penetrations.3 Our selection of an initial entry point, and thus ring and arc angle on the stereotactic frame, takes into account an optimal trajectory in coronal and sagittal planes that will allow for detection of relevant surrounding physiological borders. In addition, the trajectory is chosen to avoid traversing sulci that harbor blood vessels. Once the entry point is defined, the burr hole is planned stereotactically to place this point at its center. If the initial trajectory is not optimal, the electrode and guide cannula can be removed and repositioned in any direction up to the limit of the burr hole (and, of course, any surface vessels). Our single-electrode approach to targeting Vim and GPi always requires more than one penetration. The effort involved in repositioning the microelectrode between trajectories will depend on one’s choice of equipment. Some electrode stages have a built in X/Y platform that allows for redirection of the microelectrode without manipulation of the stereotactic frame. Although the X/Y microstage is more accessible to the surgeon, it requires a second pial penetration and displacement of the whole tract as opposed to a slight alteration in the ring angle for changes in Y and alterations in the arc angle for changes in X (Fig. 15–1). As such, adjusting the X, Y, Z, arc, and ring is less likely to shift the tract into a sulcus. Moreover, because the plane of the X/Y microstage is not likely to be parallel with the AC-PC line, the microstage also creates a theoretical concern. Moving the X/Y stage (Θ stage) creates a shift in the target that is not equal to Θ stage, but rather related by the relationship; Θ target = Θ stage /sin Θ, where Θ = ring angle. Although the error that this introduces is not likely to be significant, it should be considered. Whether or not an X/Y stage is employed, the guide cannula that directs the passage of the electrode through the most superficial regions of the brain is removed and reinserted in the new position. FIGURE 15–1 Diagram comparing the shift in target when adjusting coordinates using the frame (left) versus using an X/Y microstage (right). Electrical stimulation through the microelectrode (microstimulation) is less focused on producing a therapeutic response than it is on generating a physiological effect to help define an anatomical/physiological map. Therefore, stimulation parameters that are the most effective for activating physiological structures are used.4 In this case, much higher frequencies are used to take advantage of temporal summation of postsynaptic potentials to drive activity across multiple synapses. We typically use up to 90 μA cathodal current given as an 800 msec train of 330 pulses per sec with 0.2 msec pulse width. Microelectrode recording constitutes only one of five fundamental stages associated with deep brain stimulation surgery, and without strict adherence to the details of any one step, a poor outcome may result. These stages include the placement of the head frame, planning of the target, exposure of the cortex, microelectrode recording, and securing the electrode. A brief summary of the other four processes is provided so that the reader may have a sense of background for our procedure. Most stereotactic head frames are compatible with the currently available planning stations. Our preference is the Leksell stereotactic head frame (Elekta, Stockholm, Sweden). The critical steps in application of the head frame include using local anesthetics, placing the base parallel to the line connecting the anterior and posterior commissures (AC–PC line) to minimize yaw, pitch, and roll, which require adjustment by the software, and ensuring that the four pins have purchase on the cranium. Because the patient will be resting on these points the entire day, any alteration in pin position will translate to targeting errors. Either a magnetic resonance imaging or a computed tomography scan is obtained with a securely placed fiducial box. Although MRI has better soft tissue resolution, CT is less susceptible to distortional artifacts. Consequently, we rely on image fusion. This strategy also allows for MR scanning to be conducted prior to the day of surgery hence facilitating an early and predictable start time for the procedure. After CT scanning, the patient is transported to the operating room. The acquired images are uploaded into a planning station. Initial anatomical targeting is performed via direct and indirect approaches. With the direct targeting approach, the GPi and subthalamic nucleus can be localized on corresponding inversion recovery and T2 images, respectively. The indirect approach involves anatomical atlases as well as formula-based targeting. Superimposition of Tailarach and Sheltenbrand-Warren atlases onto patient anatomy can be achieved quite easily using computer software (Frame Link, Sofamor Danek-Medtronic), helping to confirm that the selected trajectory passes through physiologically distinct structures. The formula-based targeting uses commonly known distances from the AC and PC. For the subthalamic nucleus, these are lateral, 12 to 13 mm; AP, 4 mm; Z, +5 mm; for the Vim from PC: lateral, 11.5 from the wall of the third ventricle; AP, +5 to 9 mm; Z, 0 mm; or the GPi from the MCP: lateral, 20 mm; AP, +2 mm; Z, +5 mm. Each entry point should be selected to avoid a trajectory that passes through a sulcus and when possible to avoid the ventricles. Additionally, by selecting an entry point ~1 cm anterior to the coronal suture for the subthalamic nucleus and GPi and at the suture line for Vim, the sagittal angle of approach will traverse appropriate superficial structures. Similarly, the entry point should be between 2 and 3 cm from the midline to avoid the medial bridging veins and avoid a lateral tract in the internal capsule. Ultimately, however, we depend on evaluating our planned electrode tract in the target view on the planning station. This provides three orthogonal planes positioned with respect to the trajectory rather than the patient’s anatomy. In this way, one can ensure the most optimal trajectory from the skull to the target. Subsequent to anatomical targeting, the patient’s head is rigidly fixed in a comfortable position anticipating a 4- to 7-hour procedure. The Leksell frame is placed on the patient, and the entry point is marked on the skin. The head is then prepped and draped, and, after generous infiltration with local anesthetic, either one or two incisions are developed. Scalp incisions can be curvilinear to accommodate the burr hole cap, or they may be made as parasagittal linear incisions that pass over the burr hole. Burr holes are made with an air drill exactly 14 mm in diameter to allow the use of the Silastic burr hole ring and cover. The dura is opened in a cruciate manner, and the pia arachnoid are bipolar cauterized to obtain absolute hemostasis. The cannula is inserted into the brain through a generous pial opening. The surgeon must observe passage of the cannula to ensure that the pia is not depressed. An insufficient pial opening may precipitate pial, subpial, or subdural bleeding. Fibrin glue is used to seal the hole during each track to prevent the egress of the cerebrospinal fluid. Our cannula is typically advanced to a point 15 mm above our anatomical target. At this time, microelectrode recording and stimulation is begun. After identifying an appropriate track and target with MER, the fluoroscopy machine is draped and brought into the field. X-ray visible crosshair targets are placed in the Leksell ring to serve as a reference point (Fig. 15–2). The electrode (Model 3387 or 3389, Medtronic, Minneapolis, MN) is placed in the micro-drive and zeroed at the edge of the first contact. The electrode is advanced to the position as recorded earlier via MER. Macrostimulation is performed to assess for clinical benefits and any stimulation-induced side effects. The electrode is then secured to the burr hole device in the notch in the Silastic ring or using the Navigus Cranial Base and Cap (Image-Guided Neurologics, Melbourne, Australia), all performed with fluoroscopic visualization. Finally, the electrodes are connected to the extension cables, which are either externalized for research testing or buried for connection to the pulse generator. Attention to sterile technique throughout the procedure is critical, and the number of individuals passing in and out of the operating suite should be minimized to reduce the risk of hardware contamination. FIGURE 15–2 X-ray visible crosshair targets placed in the Leksell ring as viewed on fluoroscopy. Contact zero of the DBS lead can be seen in the center ring of the bullseye. In general, our MER setup involves the use of commercially available platinum-iridium microelectrodes (Impedance: 300–600 kOhm at 1 kHz), bandpass filters set 500 Hz to 50 kHz, a constant-current stimulus isolation unit, and a visual display and high-quality audio monitor (with infrared headphones for backup in case of difficulties with feedback or excessive ambient noise). We prefer the platinum-iridium electrodes to tungsten, as their tips are more resistant to microstimulation-induced corrosion. We favor our custom hydraulic microdrive, which allows for almost continuous sampling of the trajectory with minimal or no movement-related artifact. Other drives using stepper motors often generate considerable “noise.” Finally, we have, to date, found limited clinical use for commercial amenities such as interspike interval histograms and raster displays, although a measure of instantaneous frequency in GPe can be helpful for discerning the two major classes of units located in that region (see below). The strategy we use in attempting to locate the optimal target site varies based on the structure targeted, reflecting differences in the type of anatomical/physiological information needed. In targeting the subthalamic nucleus, for example, the goal of the initial microelectrode penetration, and that of any subsequent trajectories, is to find the optimal trajectory through the nucleus. In contrast, when targeting the Vim nucleus of the thalamus or the GPi, the purpose of the initial penetration(s) is to identify the anatomical boundaries of the structures to be avoided. These include the internal capsule and optic tract for GPi. For Vim targeting, in addition to the internal capsule, one must avoid the tactile sensory relay of the Vc nucleus of the thalamus. When targeting the subthalamic nucleus, our goal is to identify an optimal tract within the nucleus, whether that requires one or multiple trajectories. The criteria we use to define an optimal location are the following: The criterion of at least 5 mm of sensorimotor subthalamic nucleus is based on technical/anatomical practicality. Given the dimensions of the subthalamic nucleus and our typical approach angles, trajectories through the thickest part of the nucleus will be between 5 and 7 mm in length. Thus, if a trajectory has at least 5 mm of sensorimotor subthalamic nucleus, it is unlikely that any practical repositioning of the microelectrode will result in capturing a greater length of the nucleus. Regarding the clinical effects of microstimulation, it should be noted that improvements in clinical response are not always seen with microstimulation and that the effects are most robustly noted with tremor, followed next by rigidity. As mentioned previously, when using the single-electrode approach, the entire trajectory, beginning in the thalamus and going through to the substantia nigra pars reticulata or internal capsule, must be studied. Although the physiologically defined criteria for optimal target location appear adequate, the difficulty resides in deciding in which direction to reposition the microelectrode trajectory if the criteria are not satisfied. Clues are provided by physiologically identifying specific structures and the depth and width of the various structures traversed by the microelectrode. Our trajectory begins 15 mm above the anatomical target (see above), which, based on our angle of approach, typically places the tip of our cannula in the reticular or anterior thalamus. From here our trajectory takes us through the zona incerta and fields of Forel (ZI/FF), the subthalamic nucleus, and into the substantia nigra pars reticulata. Landmarks to be noted along the tract include the bottom of the thalamus, the width of the ZI/FF region, the height at which the subthalamic nucleus is encountered, the amount of the subthalamic nucleus traversed, and the distance between the bottom of the nucleus and the top of the SNr. Sensorimotor testing usually begins when neuronal activities characteristic of the subthalamic nucleus are encountered and is performed at each location where a neuron is encountered, provided it is at least 0.4 mm past the last neuronal recording site. The 0.4 mm criterion serves two purposes. First, it would be impractical to record at shorter intervals considering the time involved. Second, it helps to ensure that the units being tested are not the same as those tested previously. In specific circumstances, sensorimotor driving of the thalamic neurons also is done when the characteristics of the neuronal activity suggest that the microelectrode tip is in the ventral thalamus pars oralis posterior for reasons described below. Structures are identified by their neuronal firing characteristics. Parameters include discharge frequency, discharge regularity, and neuronal density (as indicated in the number of different extracellular action potentials recognized in the recording at a single site or the distance traversed before an active site is encountered). Parameter characteristics are summarized in Table 15–1, and representative patterns are shown in Figure 15–3. It is important to recognize sampling issues. For example, whereas the average discharge frequency of the subthalamic nucleus appears high because of the density of neurons often recorded at a single site, there are in fact ranges of frequencies. It is probable that some recording sites will be encountered whose neurons discharge at a lower frequency or where the density is low, giving the impression of a lower frequency. Similarly, not all neurons in a recording site within the sensorimotor subthalamic nucleus may be driven during sensorimotor testing. Overall, multiple recording depths must be studied to draw sound interpretations.
Target Selection Using Microelectrode Recording
KENNETH B. BAKER, NICHOLAS M. BOULIS, ALI R. REZAI, AND ERWIN B. MONTGOMERY, JR.
Approach to Microelectrode Recording
Overview of Stereotactic DBS Surgical Technique
Microelectrode Recording
Targeting the Subthalamic Nucleus
Mapping
Neupsy Key
Fastest Neupsy Insight Engine