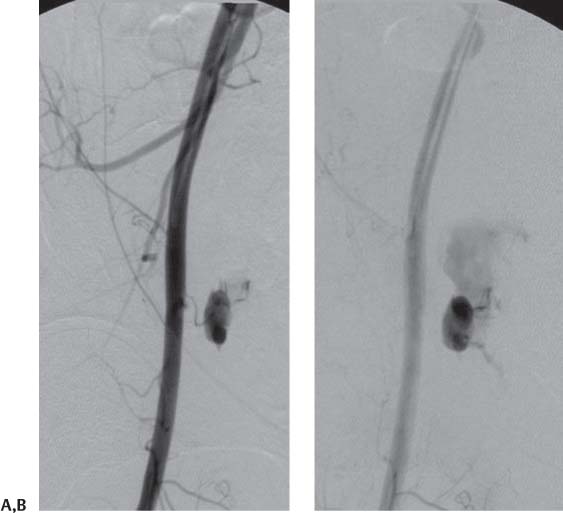
Chapter 12 The endovascular techniques used for the revascularization of intracranial stenosis have evolved over the past two decades. Initially, the procedure was limited to angioplasty alone using devices designed for the coronary circulation. As coronary interventions transitioned from angioplasty alone to angioplasty and stenting using balloon-expandable coronary stents, intracranial interventions followed suit. Although balloon-expandable coronary stents provided better luminal gain than angioplasty alone, these results were achieved at the expense of higher rates of procedural complications. In 2005, the Gateway-Wingspan system was granted Food and Drug Administration (FDA) approval under a humanitarian device exemption (HDE) for intracranial angioplasty and stenting. The Gateway-Wingspan system (Boston Scientific, Natick, MA) represents a hybrid technique that combines aspects of traditional angioplasty and primary stenting. The technique was designed to maximize safety but at the same time optimize the luminal gain achieved during the procedure. The present review will focus on the technical aspects of intracranial angioplasty and stenting using the Gateway-Wingspan system. The Warfarin and Aspirin in Symptomatic Intracranial atherosclerotic Disease (WASID) Study and the subsequent subset analyses served to identify those patients with symptomatic intracranial atherosclerotic disease (ICAD) who were at the highest risk for recurrent stroke on medical therapy.1,2 Similarly, these data serve to guide the interventionist with respect to the selection of those patients who stand to benefit the most from endovascular treatments. On the basis of these data, we can outline several important considerations for patient selection for intracranial percutaneous transluminal angioplasty and stenting (PTAS): Intracranial angioplasty and stenting must be performed with adequate platelet inhibition, which is typically achieved using two oral agents (aspirin and clopidogrel) administered prior to the procedure.5 Aspirin has a very fast onset of action and if administered even a few hours prior to the procedure in standard doses (325 mg by mouth per day) will yield adequate platelet inhibition. We typically start aspirin at least 24 hours prior to the procedure and continue it indefinitely after treatment. The effects of aspirin can be considered to be irreversible for the lifespan of the affected platelet, requiring the endogenous generation of new platelets or the administration of exogenous platelets to reverse its activity.5 Clopidogrel (Plavix; Bristol-Myers Squibb, New York, NY) is a prodrug, which requires hepatic metabolism to become active. As such, the onset of platelet inhibition with clopidogrel is considerably slower and to some extent more variable than with aspirin. Given that symptomatic intracranial atherosclerosis represents a disease that should be addressed very shortly after the patient comes to clinical attention, it is important to be able to achieve adequate platelet inhibition quickly so that revascularization can be undertaken promptly. A growing body of literature from interventional cardiology has indicated that this is best achieved using a one-time 600-mg loading dose of clopidogrel. In comparison to other clopidogrel dosing regimens, this loading dose is associated with lower rates of resistance, lower rates of clinical events, and no greater incidence of bleeding complications in patients undergoing percutaneous coronary interventions (PCI). We typically give patients this 600-mg loading dose the day before the anticipated intervention. Similar to aspirin, the effects of clopidogrel can be considered, for all intents and purposes, to be irreversible for the life span of the affected platelet.6–9 In some instances, patients have not been adequately pre-treated with aspirin and/or clopidogrel, or preoperative testing has shown them to be nonresponsive or suboptimally responsive to these medications. In these cases, platelet inhibition can be rapidly and adequately achieved with the IIb/IIIa receptor antagonists (abciximab; Reopro, Centocor Ortho Biotech, Inc., Horsham, PA), eptifibatide (Integrilin, ScheringPlough Corp., Memphis, TN) or tirofiban (Aggrastat, MGI Pharma, Inc., Bloomington, MN) administered either intravenously or intraarterially. There are no neuroendovascular studies to support the use of these agents as adjuncts in intracranial stenting. Correspondingly, the dosing and route of administration of these agents is based primarily on extrapolations from the cardiology literature and clinical experience. We have typically administered these agents in divided doses (3–5 mg) intraarterially during the case through the guiding catheter. Serial measurements of the level of IIb/IIIa receptor inhibition can be obtained with the goal of achieving 70% receptor inhibition by the end of the case.5 Assays designed for the verification of the efficacy of the administered antiplatelet medications, although controversial, have been increasingly incorporated into the practice of interventional cardiology and more recently, interventional neuroradiology. Although there are a myriad of available tests to assess the level of platelet inhibition, the most widely used is probably the commercially available VerifyNow system (Accumetrics, San Diego, CA), which is a point-of-care test that can be used to measure responsivity to aspirin, clopidogrel, and the IIb/IIIa antagonists.10,11 To perform intracranial angioplasty and stenting successfully, each step of the procedure must be performed without complication. As with any procedure, this is best accomplished by proceeding in a protocolized manner, which is performed the same way each time. The institution of a systematic approach allows the operator (and the ancillary staff) to develop a routine approach to the case and apply a more focused concentration to the critical aspects of the case. Most intracranial stenting cases are performed through a common femoral artery access. Femoral access is best achieved for these cases with a micropuncture kit (e.g., Cook Medical, Indianapolis, IN) using a careful single-wall puncture technique. The dual antiplatelet agents and procedural heparinization create an unforgiving scenario for any femoral access complications. Multiple arterial punctures during attempted access or a “double-wall” arterial puncture can create significant bleeding complications. Immediately after groin access is secured and a sheath is in place, we evaluate the puncture site with an angiogram performed in the ipsilateral oblique projection. This projection demonstrates the arterial entry site of the sheath into the femoral artery to best advantage. This information allows the operator to know immediately that it is safe to heparinize the patient and that at the conclusion of the case it will be safe to use an arterial closure device. Performing this simple step prior to proceeding with intracranial stenting can reduce the chances of fully heparinizing the patient while there is active bleeding at the groin puncture site (Fig. 12.1). Fig. 12.1 Puncture site evaluation. Early (A) and late (B) angiographic images over a right femoral puncture site demonstrate active extravasation from a right femoral pseudoaneurysm. The patient had had several previous angiograms and had developed a large right femoral pseudoaneurysm that extended into the retroperitoneum. The aneurysm was inadvertently perforated during femoral access for intracranial stenting. The patient had been on aspirin and clopidogrel and was loaded with heparin during the procedure. An abrupt drop in blood pressure during the procedure prompted imaging of the femoral puncture site. The procedure was aborted, and the femoral pseudoaneurysm was ultimately secured through a combination of open surgical exploration and placement of an endovascular stent graft from a contralateral access. As soon as femoral access is secured and the puncture site has been demonstrated to be intact, we fully load the patient with heparin (70–80 U/kg, targeting a procedural activated clotting time [ACT] of 250–300 s). Loading the patient with heparin as soon as access is secure allows the operator to perform the entire diagnostic portion of the procedure under full heparinization. In addition, this avoids having to wait to achieve a therapeutic level of heparinization later in the procedure. If the initial bolus does not achieve a therapeutic ACT, the operator can then use the time that elapses during the diagnostic angiography to administer additional boluses to achieve the level that is ultimately required to proceed with the intervention. Diagnostic angiography is performed using a standard 5F catheter. When the patient has had complete vascular imaging, the catheter can be selected specifically for the catheterization of the targeted cervical vessel. When the right carotid circulation is the target, we prefer the H1 catheter (Cook Medical). When the left carotid or left vertebral circulations are targeted, we typically start with the Vertebral (VER; Cordis Corp., Warren, NJ). When the left carotid is backward facing, often the Davis (DAV, Cook Medical) can be useful for direct catheterization prior to moving onto a Simmons-2 (Cordis Corp.). Once the proximal cervical segment of the target artery is catheterized, we perform cervical angiography before proceeding with intracranial runs. An evaluation of the cervical anatomy allows the operator to select the guiding catheter platform, which will be used to complete the case. Also, the size and tortuosity of the cervical artery may allow the operator to preemptively prepare and administer an anti-spasmodic medication (e.g., nicardipine, verapamil, nitroglycerin) to avoid flow-limiting vasospasm and an iatrogenic arterial injury. The guiding catheter should be positioned at the level of the skull base whenever possible. After the cervical anatomy is interrogated, the catheter is positioned within the proximal internal carotid or vertebral artery so that selective intracranial angiography can be performed. A standard posteroanterior (PA) and lateral branch vessel arteriogram must be done initially such that the operator will be able to exclude procedural thromboembolic complications at the conclusion of the procedure (Fig. 12.2). Next, high-magnification images of the target lesion demonstrating the stenosis to best advantage should be obtained (Fig. 12.2). The goal of the operator should be to optimally show the patent, stenotic residual lumen so that it can be successfully navigated with the microcatheter and microwire. The most stenotic view of the lesion provides the “truest” measurement of the actual stenosis. Once defined, these views will represent the working angles for intracranial angioplasty and stenting. After the working angles have been defined, all measurements of the intracranial vessel should be performed. The most critical measurements are the vessel diameter (proximal and distal to the lesion) and the length of the lesion. These measurements will determine the selection of the angioplasty balloon and stent.
Technical Aspects of Intracranial Angioplasty and Stenting
 Clinical Decision Making in Angioplasty and Stenting
Clinical Decision Making in Angioplasty and Stenting
 High-grade (70–99%) intracranial stenosis: Patients presenting with 70 to 99% stenosis had a markedly higher ipsilateral stroke risk while on medical therapy than those with less severe stenosis (50–69%). For patients presenting with a qualifying event of stroke, the ipsilateral recurrent stroke rate was 24.6% over 2 years (in comparison with only 11.2% in those patients with lower-grade stenosis).1,3 Thus, with the existing endovascular technology, it is very feasible that intracranial PTAS (if added to standard medical therapy) could provide a better secondary prevention of ipsilateral stroke in patients with high-grade stenosis. It is much less likely that a significant benefit would be provided to those with lower grades of stenosis.
High-grade (70–99%) intracranial stenosis: Patients presenting with 70 to 99% stenosis had a markedly higher ipsilateral stroke risk while on medical therapy than those with less severe stenosis (50–69%). For patients presenting with a qualifying event of stroke, the ipsilateral recurrent stroke rate was 24.6% over 2 years (in comparison with only 11.2% in those patients with lower-grade stenosis).1,3 Thus, with the existing endovascular technology, it is very feasible that intracranial PTAS (if added to standard medical therapy) could provide a better secondary prevention of ipsilateral stroke in patients with high-grade stenosis. It is much less likely that a significant benefit would be provided to those with lower grades of stenosis.
 Early treatment (within days to weeks) after the qualifying event (QE): Similar to symptomatic cervical carotid stenosis, in symptomatic ICAD, most of the risk for recurrent stroke after a qualifying event (either transient ischemic attack [TIA] or stroke) is incurred over the first few weeks after presentation. When patients with symptomatic high-grade stenosis were enrolled in WASID within 30 days of their QE, the stroke risk over the next year was 22.9%, with most of these events occurring within the first few weeks after enrollment. When patients were enrolled after 30 days, their stroke risk was only 9%.2 Therefore, endovascular revascularization, if performed, needs to be undertaken soon after the patient presents with symptoms. Although the labeled indication for Wingspan specifies that it is for use only in patients with symptomatic intracranial atherosclerotic stenosis, which is “refractory to medical therapy,” the WASID results suggest that waiting for a second event to develop may be ill-advised. In WASID, patients who presented with a QE while on antithrombotic therapy (e.g., aspirin, clopidogrel, warfarin) were at no higher risk to reach primary endpoint than those who were not on antithrombotic therapy at the time of their QE.4 Thus, in waiting for a second event to occur, it is entirely possible that the “time window” for the greatest potential benefit of revascularization could be missed altogether in most patients.
Early treatment (within days to weeks) after the qualifying event (QE): Similar to symptomatic cervical carotid stenosis, in symptomatic ICAD, most of the risk for recurrent stroke after a qualifying event (either transient ischemic attack [TIA] or stroke) is incurred over the first few weeks after presentation. When patients with symptomatic high-grade stenosis were enrolled in WASID within 30 days of their QE, the stroke risk over the next year was 22.9%, with most of these events occurring within the first few weeks after enrollment. When patients were enrolled after 30 days, their stroke risk was only 9%.2 Therefore, endovascular revascularization, if performed, needs to be undertaken soon after the patient presents with symptoms. Although the labeled indication for Wingspan specifies that it is for use only in patients with symptomatic intracranial atherosclerotic stenosis, which is “refractory to medical therapy,” the WASID results suggest that waiting for a second event to develop may be ill-advised. In WASID, patients who presented with a QE while on antithrombotic therapy (e.g., aspirin, clopidogrel, warfarin) were at no higher risk to reach primary endpoint than those who were not on antithrombotic therapy at the time of their QE.4 Thus, in waiting for a second event to occur, it is entirely possible that the “time window” for the greatest potential benefit of revascularization could be missed altogether in most patients.
 Premedication
Premedication
Aspirin
Clopidogrel
IIb/IIIa Receptor Antagonists
 Procedure
Procedure
Access
Access Technique
Evaluation of Femoral Access
Immediate Heparinization
Diagnostic Angiography
Catheter Selection
Cervical Artery Evaluation
Cerebral Angiography
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree