Needle
Comparison
Advantages
Disadvantages
Length
Short
Children
–
Long
Adults or obese patients
Procedure more difficult
Diameter
Small
Less complications, pain, discomfort, blood contaminations, medication, and medical assistance
Slow flow, longer collection time, more failures, and more training/practice needed
Large
Fast flow, shorter collection time, and less failures
More complications, large perforations, and higher risk of contaminations
Type
Cutting bevel
Feeling of penetration through skin
More complications, medication, medical assistance, and costs
Atraumatic
Reduced complication risk and less costs, medication, medical assistance, and traumatic taps
Low CSF flow, longer collection time, more failures and attempts, and no feeling of penetration through skin
Length of the Needle
Short needles are used for neonates and children, whereas longer spinal needles are used in adults and in obese patients. The procedure is more difficult with longer needles because the needles will typically be more flexible and consequently often divert off course during the procedure (Wright et al. 2012). Therefore, standard needles should be used where possible and, if necessary in case of a dry tap, the needle should be advanced further as required to obtain CSF.
Diameter of the Needle
There are many considerations to use needles with a small or a large diameter, in gauge. Small-diameter needles yield less complications after the procedure, less pain and discomfort for the patient, and lower blood patch rates as PLPH is less frequent (Hatfield et al. 2008; Lambert et al. 1997; Flaatten et al. 1998; Wilkinson and Sellar 1991; Aamodt and Vedeler 2001). Otherwise, they produce longer collection times and more failures (Tourtellotte et al. 1972; Crock et al. 2014; Ginosar et al. 2012). Another disadvantage of a smaller diameter, reported by medical staff during questionnaires in different institutions, is the need for more training during the medical education, as well as into practice to avoid the problem of failures and to increase confidence in using these needles (Tung 2013; Stendell et al. 2012; Birnbach et al. 2001; Moller et al. 2013). Considerations are vice versa for larger-bore needles.
In a total of 19 previous studies, from 1970 to 2014, different sizes of needles were compared (Table 4.2). Two study groups detected no differences in the compared needle sizes (20G vs. 22G, 23G vs. 25G) (Hammond et al. 2011; Kim and Yoon 2011); three other articles described a positive effect, such as faster collection time and less failures, when larger-bore needles were used (Ellis et al. 1992; Ready et al. 1989; Kokki and Hendolin 1996), whereas the majority of studies suggest the use of smaller-diameter needles (Hatfield et al. 2008; Lambert et al. 1997; Flaatten et al. 1998; Wilkinson and Sellar 1991; Aamodt and Vedeler 2001; Muller et al. 1994; Kleyweg et al. 1998; Vilming et al. 2001; Tourtellotte et al. 1972; Kaukinen et al. 1981; Crock et al. 2014; Lowery and Oliver 2008; Kovanen and Sulkava 1986; Palmers et al. 2002; McConaha et al. 1996; Tung 2013; Stendell et al. 2012). The articles reported a lower incidence of PLPH, low back pain, and discomfort and consequently a reduced use of medical assistance and medication after the procedure, when using small-diameter needles. Needles with a smaller diameter are also normally used to reduce the risk of PLPH and to avoid blood contaminations, defined as >5/μL red blood cells in the first tube of CSF collected (Armon et al. 2005).
Table 4.2
Comparison between needle diameters in articles published between 1970 and 2014
Conclusion | Comparison between needle diameters (gauge) | Reasons for choosing a needle diameter | References |
|---|---|---|---|
No difference in performance between needle sizes | 20 vs. 22 | = PLPH, traumatic tap incidence | Hammond et al. (2011) |
23 vs. 25 | = PLPH, low back pain, attempts | Kim and Yoon (2011) | |
Large-bore needles are preferred | 20 vs. 22 | ↓ Collection time, CSF pressure measurement | Ellis et al. (1992) |
22 vs. 25 | ↓ Leakage | Ready et al. (1989) | |
25 vs. 29 | ↓ Collection time, failures, PLPH | Kokki and Hendolin (1996) | |
Small-bore needles are preferred | 20 vs. 22 | ↓ PLPH, complaints, blood patch rates | |
↑ Collection time | |||
22 vs. 26 | ↓ PLPH, pain | ||
↑ Practice | |||
22 vs. 25 | ↓ PLPH interval, low back pain | ||
20 vs. 22 vs. 23 | ↓ PLPH | Kovanen and Sulkava (1986) | |
22 vs. 24 | ↓ PLPH | Palmers et al. (2002) | |
22 vs. 29 | ↓ PLPH, failures | McConaha et al. (1996) | |
25 vs. 26 vs. 27 | ↓ PLPH, blood patch rates | Lambert et al. (1997) | |
19 vs. 20 vs. 22 | ↓ PLPH | Flaatten et al. (1998) | |
22 vs. 25 vs. 27 | |||
Questionnaire to medical institutions, favors the small-needle type | 20 vs. 22 | ↓ PLPH, costs | Tung (2013) |
22 vs. 24 | ↓ Complaints | Stendell et al. (2012) |
In diagnostic context, LPs are often performed with larger-gauge needles (20–22G), whereas for therapeutic purposes, the needles are more narrow (25–27G) (Ginosar et al. 2012; Boon et al. 2004).
Reasons for using large-bore spinal needles include the increased speed in obtaining CSF samples by passive flow and the shorter time required to equilibrate the CSF pressure, when using a manometer. For CSF pressure measurement, needles smaller than 22G (thus >22G) are not suitable. Moreover, it is not recommended to use the smallest needle available because more technical difficulties occur, leading to failures, and because the duration of the procedure will be prolonged through the slow flow with these small spine needles (Flaatten et al. 1989). On the other hand, large needles are also not preferred as they produce large dural perforations with a higher risk of PLPH and contaminations. Needles used in children are selected based on the same criteria used for adults as described above.
In conclusion, a balance between the risk of PLPH, procedure duration, flow, and technical failure has to be considered for each patient individually (Turnbull and Shepherd 2003). The smallest needle as possible is recommended; however, longer sampling time and more failures should be taken into account for small-diameter needles, though with a reduced risk of PLPH, low back pain, and less discomfort. Nevertheless, in most centers, a 22G needle or larger needle diameter is used (Stendell et al. 2012; Birnbach et al. 2001). Once the practitioner is more confident, by often usage, the use of smaller needle types should be considered, which will lead to less failures and an easier procedure (Tung 2013).
Design of the Needle
To date, different designs of needles are available; Quincke-type needles (Spinocan® and Yale™) are the standard needles with a cutting bevel and the orifice at the needle tip. Whitacre™, Sprotte®, Atraucan®, Pencan®, and Pajunk® needles are noncutting, pencil-point, or atraumatic needles (Fig. 4.1).
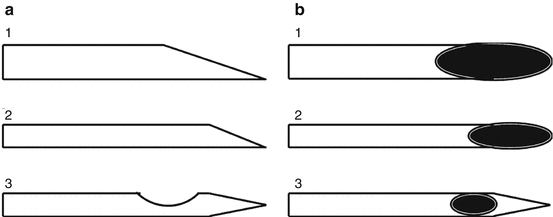

Fig. 4.1
Schematic representative of lateral (a) and superior (b) aspects of the tips of spinal needles. 1 standard large-bore beveled needle, 2 standard small-bore beveled needle, and 3 atraumatic small-bore needle
Cutting bevel needles are still the standard needles mostly used in practice, because of the disadvantages of the atraumatic needles, such as low CSF flow and longer sampling time, little availability of these needles, less practical experience, a high failure rate, and the fact that when using local anesthetics, due to the thick introducer needle that penetrates the skin, it cannot be felt as well as with the standard needles when the dura is penetrated (Tung 2013; Stendell et al. 2012; Birnbach et al. 2001; Moller et al. 2013; Sharma et al. 1995). Needle types were compared in 35 studies between 1970 and 2014 (Table 4.3). No difference was reported in two studies (Quincke vs. Whitacre™) by comparing transdural fluid leakage and vascular trauma associated with blood contamination in CSF (Ready et al. 1989; Knowles et al. 1999). Three studies investigated the incidence of post-LP complications, such as PLPH and low back pain, and found no differences for all comparisons (Yale™ vs. Sprotte®, Spinocan® vs. Whitacre™, Quincke vs. an atraumatic needle type) (Aamodt and Vedeler 2001; Lenaerts et al. 1993; Luostarinen et al. 2005).
Table 4.3
Comparison between needle types in articles published between 1970 and 2014
Conclusion | Comparison between needle types | Reasons for choosing a needle type | Comments | References |
|---|---|---|---|---|
Atraumatic needle favors compared to the cutting bevel needle | Quincke vs. Sprotte® | ↓ PLPH, post complaints, nausea/vomiting, costs, medication, medical assistance, traumatic taps | Atraumatic needles less available. CSF IgM concentration is same as in serum | |
↑ Training | ||||
Quincke vs. Whitacre™ | ↓ PLPH, post complaints, blood patch rates | |||
Quincke vs. Sprotte® vs. Whitacre™ | ↓ PLPH | Minor epithelial cells, fewer and smaller cell clusters attached to needle | ||
Quincke vs. Spinocan® Sprotte vs. Whitacre | ↓ PLPH | Carson and Serpell (1996) | ||
Quincke vs. Sprotte® vs. Pajunk® | ↓ PLPH, ↑ failure rate | Thomas et al. (2000) | ||
Quincke vs. Pencan® | ↓ PLPH, low back pain | Interval of PLPH is reduced | Lowery and Oliver (2008) | |
Yale™ vs. Pencan® | ↓ PLPH | Kokki et al. (2000) | ||
Questionnaire to medical institutions, favors the atraumatic needle type | Quincke vs. Sprotte® | ↓ PLPH, costs | Tung (2013) | |
↑ Practice, training | ||||
No difference in performance between needle types | Quincke vs. Whitacre™ | = In RBC count, leakage | ||
Yale™ vs. Sprotte® | = PLPH, traumatic tap | Incidence of PLPH and traumatic tap is the same for both needle types | Lenaerts et al. (1993) | |
Spinocan® vs. Whitacre™ | = PLPH, low back pain | Incidence of PLPH and low back pain is the same for both needle types | Luostarinen et al. (2005) |
Nevertheless, in the 35 published articles, most studies recommended atraumatic needles as the best needle to perform LPs, when different needle types were compared (Table 4.3) (Alcolea et al. 2014; Hatfield et al. 2008; Lambert et al. 1997; Hammond et al. 2011; Muller et al. 1994; Kleyweg et al. 1998; Lowery and Oliver 2008; Palmers et al. 2002; Braune and Huffmann 1992; Ohman et al. 1995; Strupp et al. 2001; Tung et al. 2012; Vakharia and Lote 2012; Davis et al. 2014; Pedersen 1996; Kokki et al. 1999; Lavi et al. 2007; Lavi et al. 2006; Puolakka et al. 2000; Quinn et al. 2013; Carson and Serpell 1996; Thomas et al. 2000; Kokki et al. 2000; Reynolds and O’Sullivan 1998; Dakka et al. 2011; Vidoni et al. 2014). Advantages of the atraumatic needles are a lower incidence of PLPH, low back pain, and nausea/vomiting than cutting bevel needles, which consequently reduce healthcare costs as less medication and medical assistance, such as blood patches, are needed after the procedure (Cruickshank and Hopkinson 1989). Furthermore, lower incidence in traumatic taps is described when atraumatic needles are used; however, more attempts and failures are reported for these needle types. No exact data is available concerning other post-LP complaints than PLPH, number of traumatic taps, or failures. The technical drawbacks of atraumatic needles can be overcome with more training, also within practice, and likewise, several authors conclude that the disadvantages seem less decisive if the reduced risk of PLPH and, therewith, the time and cost savings for the healthcare system are taken into account (Strupp et al. 2001; Tung et al. 2012; Linker et al. 2002; Peskind et al. 2005; Arendt et al. 2009), which especially holds true for younger patients who have a higher risk of PLPH than the elderly.
Conclusion
In conclusion, head-to-head studies are in favor of atraumatic-type and small-diameter needles with regard to the lower incidence of PLPH. Recently published consensus-based recommendations for preanalytical issues on AD and Parkinson’s disease (PD) CSF biomarker analysis recommend to use 25G atraumatic needles (del Campo et al. 2012). However, at least in Europe, many centers keep using small to medium cutting bevel-type needles, arguing that a higher CSF flow and, thus, shorter sampling time with a lower failure rate are important, with still very acceptable complication rates. Nevertheless, 25G atraumatic needles are recommended based on the JPND (the EU Joint Programme – Neurodegenerative Disease Research) “BIOMARKAPD” consortium on the standardization and harmonization of biomarkers for AD and PD (Engelborghs et al. submitted).
4.1.2.2 Complementary Equipment That Guide the LP Procedure
Difficulties in CSF sampling can occur, such as hemorrhagic CSF in case of a traumatic LP or a misplaced needle due to incorrect estimation of the anatomical landmarks in case of obese patients or spinal malformations. A standard LP procedure is mostly successful; nevertheless, in some patients, the execution of the technique is difficult. Alternatives to surface landmark-guided LP are fluoroscopy and ultrasound guidance (Table 4.4).
Table 4.4
Advantages and disadvantages of equipment that guide the LP procedure
Type of equipment | Advantages | Disadvantages |
|---|---|---|
Fluoroscopy | Real time, less traumatic taps due to a single pass of the needle | Radiation exposure and requirement of a radiologist |
Ultrasound | More certainty and no radiation | Not real time, asset is inversely related to BMI |
Fluoroscopy shows the bone structures of the lumbar spine and provides real-time information about the precise position of the needle as it is being inserted (Eskey and Ogilvy 2001). During fluoroscopy, it is almost always possible to access the subarachnoid space with a single pass of the needle, whereas LPs at bedside rely on manual and mental estimation of the bone anatomy to guide the needle. While fluoroscopy is rarely needed to access the subarachnoid space with a spinal needle, it is routinely used for difficult cases in which bedside LP has failed. Traumatic taps are reduced by minimizing disruption of vascular structures, which is best accomplished by accessing the subarachnoid space with a single pass of the needle and avoiding the anterior epidural venous plexus entirely; this is easily done with the use of fluoroscopy. Disadvantages of this technique are the radiation exposure for the patient and the requirement of a radiologist to perform the procedure. To avoid these problems of fluoroscopy, ultrasound can be used to identify pertinent landmarks in those patients whose anatomical structures are difficult to palpate or whose body mass index (BMI) is in the obese category (Stiffler et al. 2007). Ultrasound may thus provide enough information to allow the physician to proceed with more certainty. However, the asset of this technique is inversely related to the patient’s BMI, and ultrasound is often used before the actual LP takes place and, thus, not in real time.
4.2 Contraindications
An LP is an extremely safe procedure when performed by an experienced or properly supervised practitioner using standard methods. Nevertheless, contraindications can appear due to fluid shifts and pressure gradient changes between CNS compartments.
Lowering of the spinal compartment pressure, by removal of CSF, can cause a caudal shift of the transtentorial or tonsillar structures from their normal position, referred to as a CNS herniation (Sinclair et al. 2009). For this reason, all patients undergoing an LP have, theoretically, a risk of cerebral herniation. In patients with normal intracranial anatomy and pressure, this risk is negligible. However, by performing an LP in patients with abnormal ICP, the lumbar CSF pressure is lowered further, allowing the raised pressure compartment above the puncture site to move along the pressure gradient and consequently cause herniation. This is different from uniformly raised ICP within the whole CNS compartment, e.g., idiopathic intracranial hypertension, where no internal pressure gradient has developed and where it is safe to perform an LP.
While structural brain imaging frequently eliminates the need for diagnostic LP by providing a firm diagnosis, such as subarachnoid hemorrhage or brain tumor, indications for diagnostic LP still remain, such as suspected bacterial, viral, or carcinomatous meningitis and meningoencephalitis, suspected subarachnoid hemorrhage in case of negative structural imaging of the brain, AD and related disorders, atypical multiple sclerosis (Stangel et al. 2013), and suspected spinal cord compression from metastatic diseases requiring myelography (Gower et al. 1987; Engelborghs 2013). For these indications, drug administration, and other diagnostic purposes, the LP is contraindicated if the risk of the procedure outweighs the potential benefit. In practice, a brain computed tomography (CT) or MRI scan is always performed prior to LP if a patient is over the age of 60 years, or immunocompromised, or has a previous CNS disease, any recent seizures, reduced consciousness, papilledema, or an abnormal neurological examination. This is why, in many centers, it is routine to perform brain imaging before an LP to exclude potential risks. In some circumstances requiring urgent medical care, the physician can decide to perform a fundoscopy only in order to rule out papilledema before proceeding to LP.
The following CT criteria should be considered as a contraindication, while they can be applied for MRI imaging too. However, it should be emphasized that imaging appearances should always be put in context of the individual case (Hasbun et al. 2001; Gopal et al. 1999).
CT evidence of unequal pressures across the falx cerebri
Unequal supratentorial pressures are found by lateral shift of the midline structures (septum pellucidum, third ventricle, pineal gland). Asymmetry of the lateral ventricles alone is not an accurate sign, since ipsilateral ventricular dilation may occur secondary to stroke, or coaptation of a frontal horn may represent a normal anatomical variant. Result of LP of unequal supratentorial pressures would most likely lead to compression of the ipsilateral temporal lobe, leading to an uncal herniation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







