Chapter 12 Temporal Bone
We’ll start from the outside and work our way inward.
EXTERNAL AUDITORY CANAL
Congenital Anomalies
Atresia and Hypoplasia
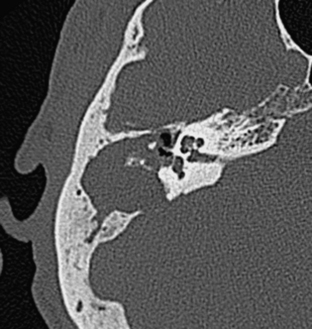
Congenital anomalies of the EAC are rather common, more so than middle ear abnormalities. The degree of congenital deformities of the EAC runs the gamut from total atresia to webs, to hypoplasia or stenosis of the EAC, to microtia (a small auricle of the ear) (Fig. 12-1 and Box 12-1).
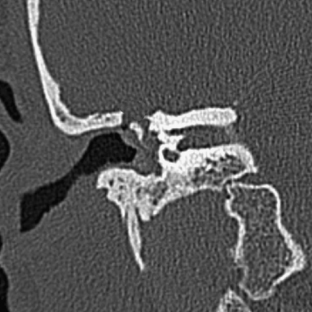
With a stenotic EAC, the slope of the EAC may be more vertically angulated. In addition, keratinous plugs and cholesteatomas may form. Rarely (11% to 30%) is there an associated anomaly of the inner ear, mainly because inner ear structures are derived from the neuroectoderm as opposed to the branchial system. In patients with EAC atresia, the facial nerve in its horizontal section may have an aberrant course in close proximity to the stapes footplate and may be anteriorly located in its descending portion. Evaluation of the facial nerve position (see Chapter 2), carotid and jugular anomalies, middle ear and mastoid air cell pneumatization, ossicular deformities, meningoencephaloceles, sigmoid sinus location, and other features is important for preoperative planning to prevent accidental surgical injury. The facial nerve may also be dehiscent in its tympanic course.
Surgery to correct the EAC is fruitless if the inner ear is nonfunctional. This must be evaluated. Surgery for external ear anomalies is difficult because it often requires grafts of bone and cartilage as well as drilling new canals. Thus, correction just for cosmetic purposes is usually delayed until after adolescence, when growth has slowed down. However, if the ear anomaly leads to learning disabilities it may be treated before schooling (Table 12-1).
Table 12-1 Checklist for Evaluating for EAC Atresia or Stenosis
| Item | Rationale |
|---|---|
| Inner ear structures | No sense fixing the outer, middle ear if no sensorineural function |
| Stapes | Implies inner ear anomaly, means implant |
| Oval and round window | Access to perilymph, endolymph |
| Middle ear space | Need place to put, repair ossicles, transmit sound |
| Facial nerve | Don’t want to injure nerve and wake patient up with facial disfigurement for rest of life |
| Ossicular anatomy | How many need to be replaced? Are there functioning joints? |
| Carotid artery, jugular vein | Makes for a bloody mess if they are anomalous and in the way |
First Branchial Cleft Cysts
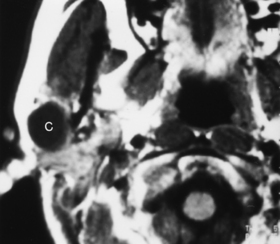
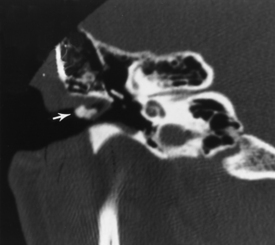
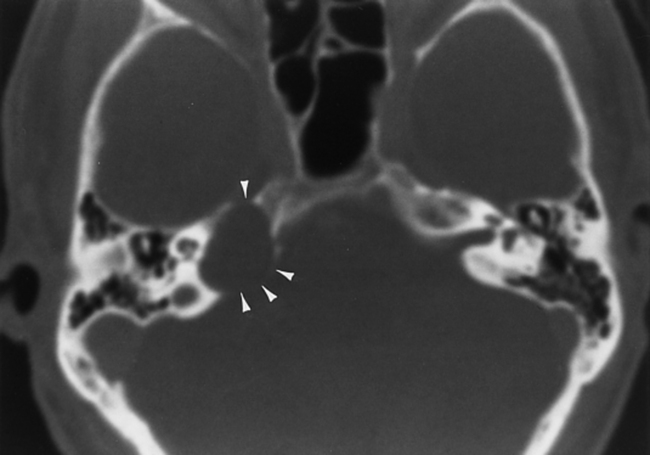
First branchial cleft cysts occur around the ear or in the neighboring parotid gland (Fig. 12-2). A fistula from a first branchial cleft anomaly may drain to the EAC at the bone-cartilage interface. Second branchial cleft cysts are 10 times more common than first branchial cleft cysts, but the external ear would be an unusual site for a second branchial cleft cyst to drain.
Calcifications of the external ear or pinna occur in a variety of congenital and acquired lesions. Box 12-2 lists these entities.
Inflammatory Lesions
Malignant Otitis Externa
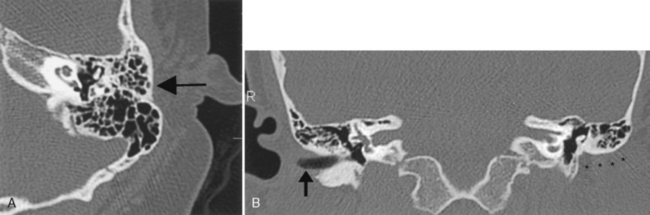
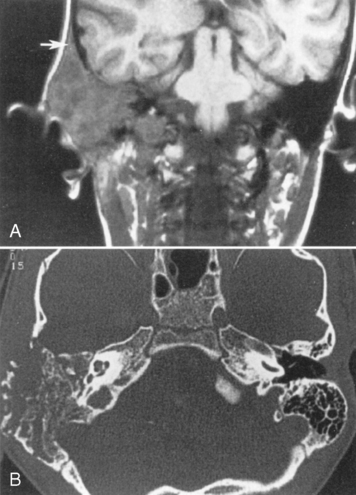
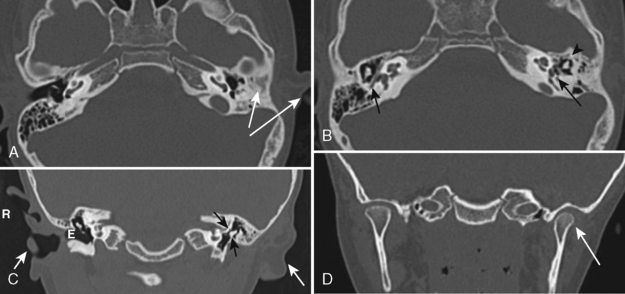
The most severe inflammatory condition affecting the EAC is malignant otitis externa, a Pseudomonas infection of the EAC seen in elderly diabetic patients (93% of cases). HIV-infected patients may also be at risk. Patients present with putrid-smelling, purulent discharges coming from their ear. The infection usually begins at the junction of the cartilaginous and bony portion of the EAC along the fissures of Santorini, which lead to the parapharyngeal space. Irrigation of the ear under pressure will drive the inflammatory process out of the EAC, leading to a potential aggressive infection extending into the infratemporal fossa, the nasopharynx, the parapharyngeal space, the adjacent bone, the temporomandibular joint, the middle and inner ear structures, and intracranially in the extradural space. Palsies of cranial nerves VI, VII, and IX through XII may reflect extension of the disease at the skull base and neural foramina. Venous sinus thrombosis is a complication. The process may mimic an aggressive neoplasm in many respects and often is difficult to control with antibiotics. CT will identify soft tissue in the EAC, bony erosion of the EAC walls, and osteitic changes at the skull base. Magnetic resonance (MR) imaging may demonstrate edema of the external ear, the parapharyngeal fat may be obliterated as the infection extends anteriorly and medially, and the tissue planes around the carotid sheath may be infiltrated (Fig. 12-3). The signal intensity of the skull base may be abnormal and the bone may enhance. Technetium-99m bone scans and gallium-67 citrate scans show uptake of radiotracers and may be useful to assess activity of disease.
Swimmer’s Ear and Surfer’s Ear
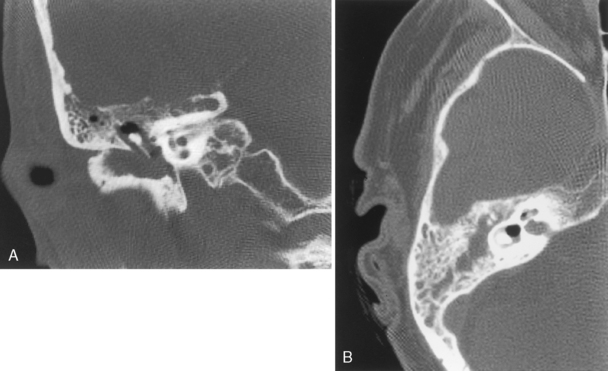
Swimmer’s ear (acute external otitis) is usually due to Pseudomonas infection. Rarely, mastoiditis or osteomyelitis may complicate swimmer’s ear. Patients who swim in cold water are prone to exostoses of the EAC (surfer’s ear). Recurrences will occur if the swimmer returns to the frozen pool or frigid oceans (Fig. 12-4). Relapsing chondritis, frostbite, and severe sunburns may affect the auricle of the ear, particularly the upper helix.
Of course, the most common EAC mass is due to benign wax buildup.
Benign Neoplasms
Benign tumors of the external ear include hemangiomas, venous vascular malformations, nevi, ceruminomas (adenomas of the ceruminous glands), polyps, and minor salivary gland tumors (included in Box 12-3). All of these present as soft-tissue masses that may expand the EAC without destructiveness. All may enhance.
Malignant Lesions
Squamous Cell Carcinomas
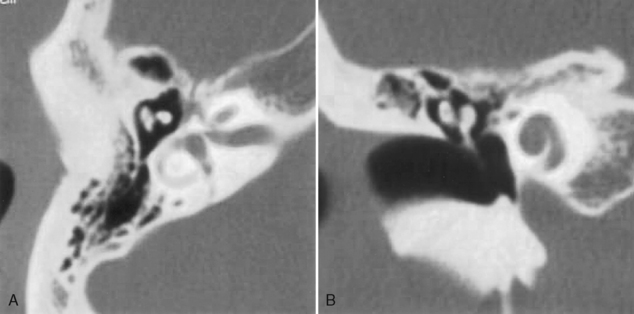
Squamous cell carcinomas of the EAC are the most common neoplastic processes in this location. This is essentially a skin cancer with the associated risk from sun exposure. The lesion may invade the middle ear and temporal bone. Cartilaginous invasion of the external ear or middle ear extension portends poor prognosis and must be treated aggressively. The 5-year prognosis for EAC squamous cell carcinomas without middle ear disease is 59%, but it is 23% if the cancer extends into the middle ear. Deep lesions in the bony canal have the worst prognosis (Fig. 12-5). Pain occurs early because of periosteal spread or extension to the temporomandibular joint, where trismus may also arise. Facial nerve involvement also occurs early in the course.
Other Skin Tumors
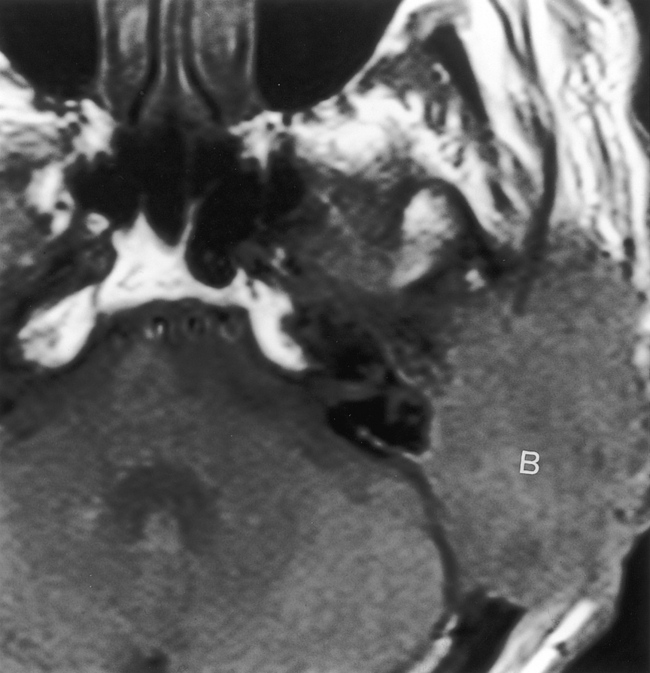
Other skin tumors such as basal cell carcinomas or melanomas may also affect the external ear in the same manner as squamous cell carcinomas. In rare cases, they develop perineural spread via cranial nerves V and VII (Fig. 12-6). Lymphatic drainage may be to intraparotid, retropharyngeal, occipital, and skull base lymph nodes. Kaposi sarcoma in HIV-positive individuals could affect the ear. Carcinomas of the parotid gland commonly invade the temporal bone and EAC, particularly if perineural growth occurs along cranial nerve VII. Adenoid cystic carcinoma of the parotid is the classic histology for this eventuality.
Pediatric Tumors
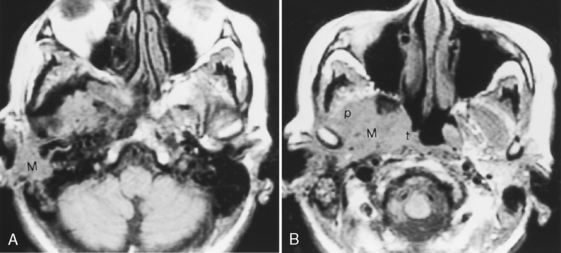
In children, rhabdomyosarcomas and lymphomas may present as external ear masses. Spread to the middle ear or along the eustachian tube is not uncommon, and in fact, tumors arising in the nasopharynx can present as ear masses because of retrograde growth along this route. Invasion of the petrous and mastoid portions of the temporal bone is not uncommon (Fig. 12-7).
THE MIDDLE EAR
Normal Anatomy
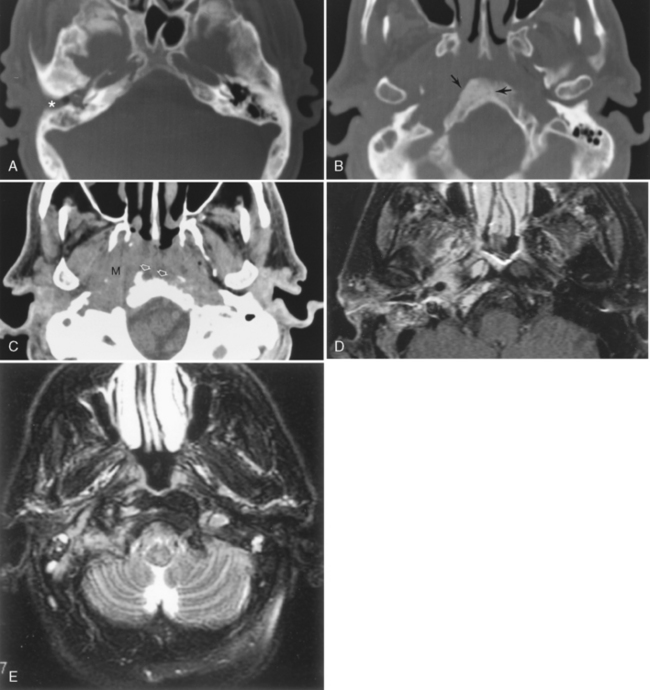
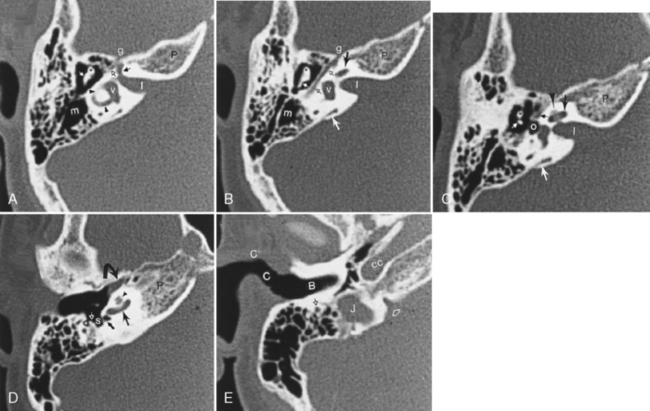
The middle ear or tympanic cavity is often divided into a superior attic or epitympanic recess, the mesotympanum at the level of the tympanic membrane, and the hypotympanum lying inferior and medial to the tympanic membrane (Fig. 12-8). Some of the contents of the middle ear cavity and eustachian tube are derived from the first branchial pouch. The first branchial arch forms the bodies of the malleus and incus and the short process of the incus. The second branchial arch forms the superstructure (capitulum and crura) of the stapes and long process of the incus, as well as the manubrium of the malleus. The first branchial pouch develops into the eustachian tube, mesotympanum, and mastoid air cells.
The footplate of the stapes covers the oval (vestibular) window. The stapedius muscle arises from the pyramidal eminence and attaches to the head of the stapes. This muscle tends to dampen sound by preventing excessive stapedial vibration. This explains the hyperacusis with seventh nerve palsies, because this muscle is innervated by the facial nerve (Fig. 12-9).
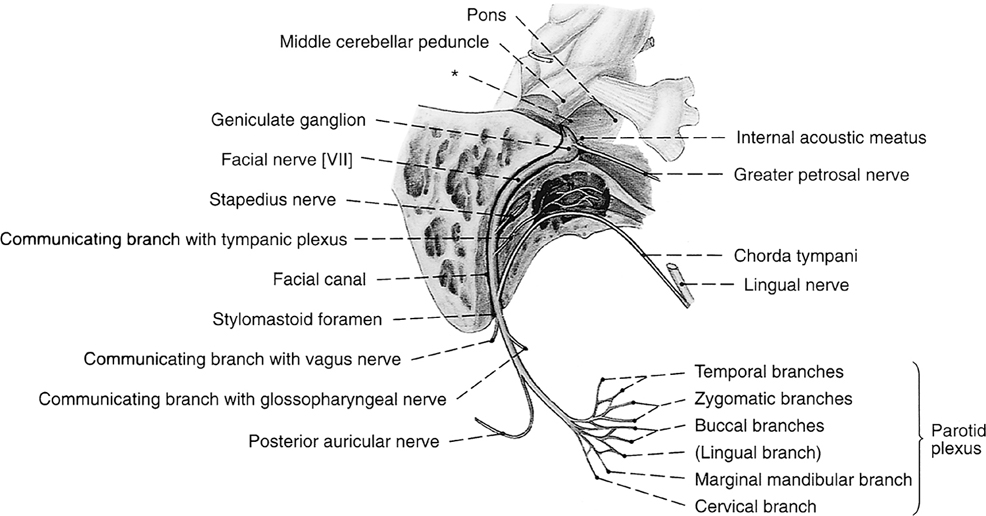
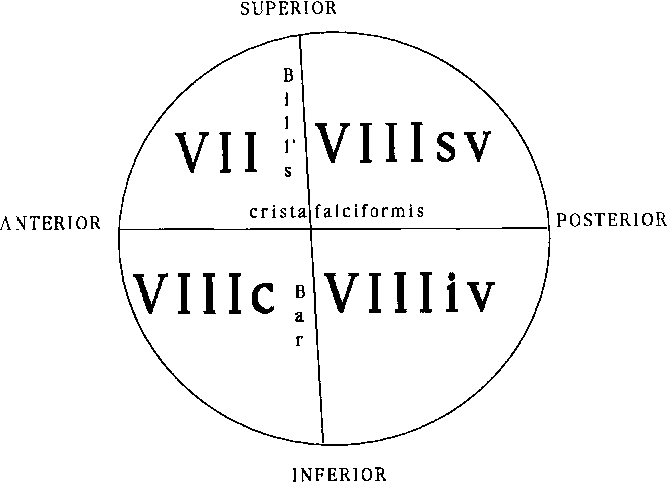
The facial nerve courses through the middle ear after entering from the internal auditory canal (IAC) (Box 12-4). The IAC is separated into superior and inferior sections by the transverse crista falciformis and into anterior and posterior quadrants by Bill’s bar (Fig. 12-10). Cranial nerve VII is found in the anterosuperior portion of the IAC. The facial nerve has a labyrinthine segment coursing anterosuperiorly and laterally from the IAC to the geniculate ganglion. The geniculate ganglion is superior to the cochlea (see Figs. 12-8 and 12-9). Here it gives off the greater superficial petrosal nerve, which innervates salivation among other things. From the geniculate ganglion, the facial nerve forms its first genu and runs posteroinferolaterally on the undersurface of the lateral semicircular canal and above the oval window niche in its horizontal (or tympanic) segment. The facial nerve then makes its second turn (the second genu) to course inferiorly in the mastoid bone in its descending (or intramastoid) segment before exiting at the stylomastoid foramen. Along its course it gives innervation to the stapedius muscle and, just above the stylomastoid foramen, to the chorda tympani for taste to the anterior two thirds of the tongue. The chorda tympani doubles back on itself, running superiorly, and reenters the mesotympanum before exiting anteriorly via the petrotympanic fissure to join the lingual nerve.
Box 12-4 Segments of Cranial Nerve VII
| Cisternal (cerebellopontine angle cistern) | |
| Intracanalicular | |
 | |
| Intraparotid |
In the inferoposterior portion of the middle ear cavity, four important structures are visible on axial scans. They are, medially to laterally, the round window niche, the sinus tympani, the pyramidal eminence, and the facial nerve recess (see Fig. 12-8). The sinus tympani and facial nerve recess are indentations in the bone; the pyramidal eminence is a bony hillock separating the two. The stapedius muscle belly and tendon lie in the pyramidal eminence. These recesses are important in that they may become a site for occult inflammatory disease after middle ear surgery.
Congenital Anomalies
Hypoplasia and Fusion
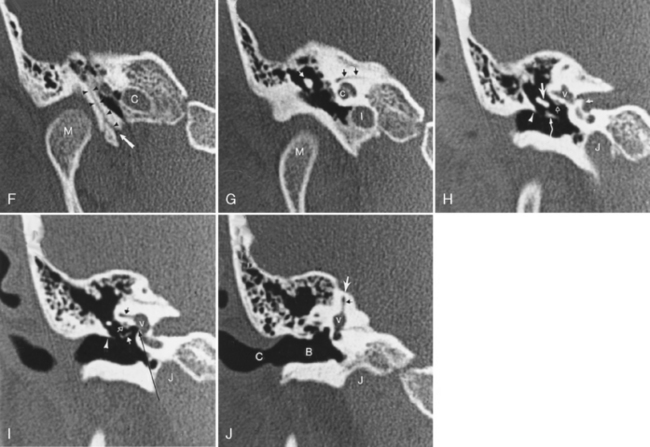
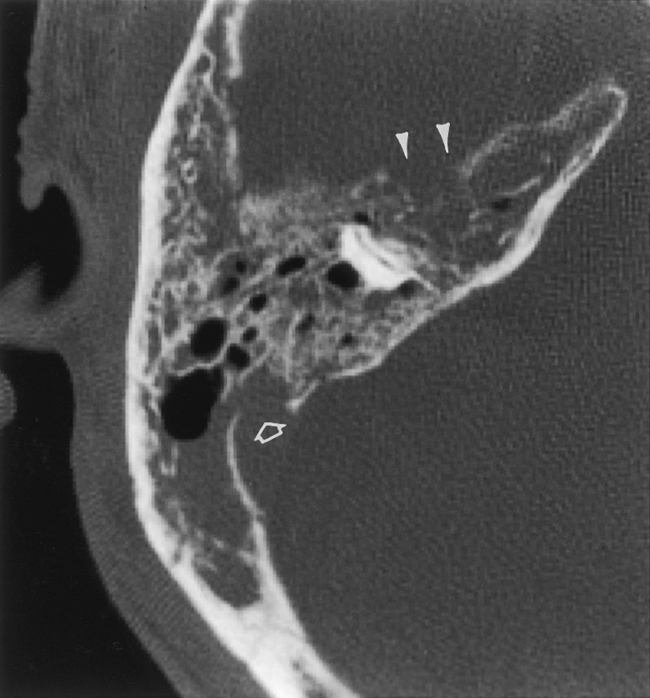
The middle ear is also a site of congenital dysplasias that may be associated with EAC stenosis or atresia (Fig. 12-11). Ossicular fusion, hypoplasia, or maldevelopment may occur and may coexist with anomalies of the facial nerve as it runs through the middle ear cavity. Isolated middle ear congenital abnormalities are not as common as those of the EAC or inner ear. When they occur in the absence of external ear anomalies, the distal incus (especially the long process) and the stapes are most commonly affected in concert, followed by the stapes alone and incus alone. These components of the ossicular chain are derived from the second branchial arch. Absence, hypoplasia, and fixation to the attic may be seen.
Epidermoids
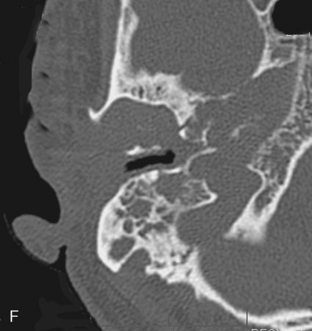
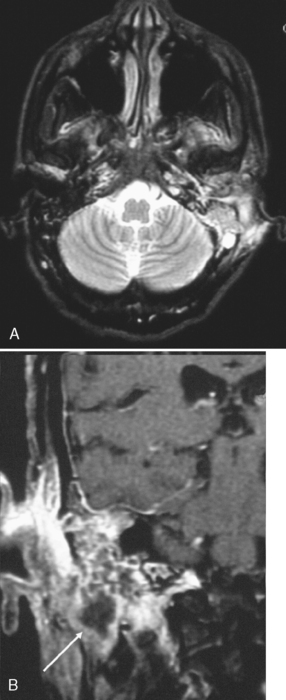
To prevent confusion with acquired cholesteatomas, use the term epidermoids rather than the older terms congenital cholesteatomas, epidermoidomas, or primary cholesteatomas. Epidermoids may arise in a variety of locations within the temporal bone (Fig. 12-12) from aberrant epithelial rests. The temporal bone is, in fact, the most common skull base site of congenital epidermoids. The classic locations for these lesions include the petrous apex, Koerner’s septum (the petrosquamosal suture), mastoid air cells, eustachian tube opening, geniculate ganglion region, middle ear-epitympanum junction, incudostapedial joint, sinus tympani, and facial nerve recess. Intracranial locations for epidermoids are in the IAC and the basal and cerebellopontine angle cisterns. These lesions are pearly white to the surgeon’s eye and are usually hypointense on T1-weighted images (T1WI) and hyperintense on T2-weighted images (T2WI). As in the brain, they are as bright as cerebrospinal fluid (CSF) on T2WI but are more intense than CSF on T1WI. They are brighter than CSF on fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted images (DWI). On CT, they appear as noninvasive, low-density, erosive, well-circumscribed lesions in the temporal bone with scalloped margins (Fig. 12-13). Although they may be whitish lesions that simulate acquired cholesteatomas, these lesions are not associated with perforation of the tympanic membrane, and the patients have no history of antecedent ear infections or previous surgeries. Presentation often is with deafness, vertigo, or facial nerve palsy. The scutum is usually intact. Epidermoids may be solid or cystic. Epidermoids do not enhance, whereas acquired cholesteatomas may enhance perip herally. Treatment for both is surgical excision.
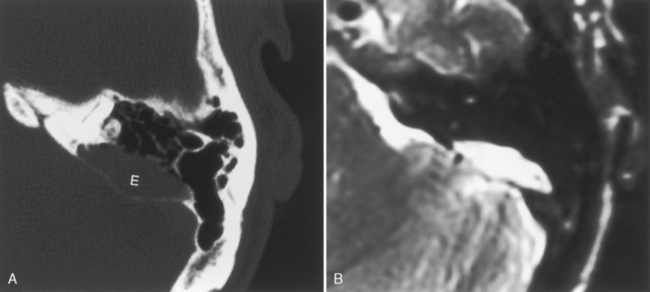
Figure 12-12 Epidermoid (E) of bone. A, A lytic oblong bone lesion is seen along the posterior margin of the top of the petrous bone. B, Note how bright the mass is on T2-weighted image. The diagnosis was an intraosseous epidermoid. Contrast this case with that of the enlarged vestibular aqueduct on computed tomography and magnetic resonance imaging (see Fig. 12-32).
Inflammatory Lesions
Otitis Media
Inflammatory disease of the tympanic cavity is common in children. Opacification of the epitympanic recess with thickening of the tympanic membrane is often seen in patients with otitis media. The most frequent causes of otitis media are Streptococcus, Moraxella catarrhalis, Haemophilus influenzae, and Pneumococcus. Obstruction of the eustachian tube from nasopharyngeal lymphoid hypertrophy caused by upper respiratory tract infections is responsible for this condition in children. Otitis media generally responds well to antibiotics. Rarely, ossicular erosions, usually a marker for acquired cholesteatoma, can occur in association with acute otitis media. When they occur, they usually affect the long process of the incus and can lead to conductive hearing defects. The erosions appear on CT as tiny, lytic, punched-out areas in the ossicle. The middle ear is filled with fluid density and intensity on CT and MR, respectively, in uncomplicated otitis media (Fig. 12-14). Although pneumolabyrinth is more commonly seen in cases of barotrauma or in the postoperative setting, occasionally you may see air in the inner ear structures with infections, presumably from a labyrinthine fistula. Gas-producing organisms may be at fault.
Ossicular disruptions may be caused by trauma or infection (Fig. 12-15). A gap of greater than 1 mm between the long process of the incus and the head of the stapes is suspicious for disruption. This finding is equally well displayed on axial or coronal sections. Usually the inflammatory causes are due to erosion of the long process of the incus. Fibrosis may ensue. Because the incus is not well suspended by the anchoring ligaments, the incudostapedial joint is most commonly affected by trauma. Therefore, dislocations and subluxations are common between the incus and stapes. Incudal disarticulation accounts for more than 80% of post-traumatic conductive hearing loss.
Mastoiditis
Infection may travel from the middle ear via the aditus ad antrum (the narrow channel connecting the middle ear cavity to the mastoid antrum) to the mastoid air cells. Mastoiditis may occur as a complication of otitis media; coalescence of mastoiditis portends a poor prognosis because it represents bony infection rather than mucositis. β-Hemolytic streptococci and pneumococci are usually the pathogens involved. CT reveals opacification of air cells; bone destruction constitutes a criterion for coalescent mastoiditis. The infection is very bright on T2WI. Occasionally, air-fluid levels within the small mastoid air cells can be seen. Middle ear or petrous apex opacification may coexist. Coalescent mastoiditis may also be a complication of cholesteatoma formation (Fig. 12-16).
Complications of acute otomastoiditis include sigmoid sinus thrombosis (see Chapter 4), thrombophlebitis, epidural abscesses, meningitis, subperiosteal abscesses, fistulas, and osteomyelitis. Cerebellar or temporal lobe encephalitis is uncommon. Bezold’s abscess is an inflammatory collection that occurs inferior to the mastoid tip as the infection spreads from the bone to the adjacent soft tissue (Fig. 12-17). It can spread down the plane of the sternocleidomatoid muscle to the lower neck.
Acquired Cholesteatomas
Long-standing otomastoiditis may result from chronic eustachian tube dysfunction. This may lead to recurrent otitis media and acquired cholesteatomas; these two entities are easier to distinguish in textbooks than in real life, where imaging characteristics may overlap (Table 12-2). Acquired cholesteatomas are erosive collections of keratinous debris from an ingrowth of stratified squamous epithelium through a perforated tympanic membrane. The critical features in identifying a lesion as a cholesteatoma are the presence of mass effect, bony erosion, or expansion (Fig. 12-18). The cholesteatoma most often arises from a perforation in the pars flaccida of the tympanic membrane. Once the pars flaccida (Shrapnell’s membrane) has been violated, the inflammatory process proceeds into Prussak’s space, which is located lateral to the middle ear ossicles but medial to the scutum in the epitympanic space. One often sees a soft-tissue mass causing erosion of the scutum and medial displacement of the malleus and incus with pars flaccida cholesteatomas. The head of the malleus and body of the incus are the areas most susceptible to erosion by a pars flaccida cholesteatoma; lysis of all the middle ear ossicles is uncommon. From Prussak’s space the lesion often spreads through the aditus ad antrum, expanding its waist as the inflammatory process proceeds into the mastoid air cells. On MR, cholesteatomas are hypointense on T1WI and intermediate on T2WI, and do not enhance, as opposed to granulation tissue (postoperative), which does enhance.
Table 12-2 Cholesteatoma versus Otitis Media
| Feature | Cholesteatoma | Otitis media |
|---|---|---|
| Middle ear opacified | Yes | Yes |
| Scutum | Eroded | Normal |
| Ossicular erosion | Yes | Infrequent |
| Ossicular displacement | Yes | No |
| Expansion of aditus ad antrum | Sometimes | No |
| Lateral semicircular canal fistula | Sometimes | Infrequent |
| Gadolinium enhancement | Rare | Rare |
| T2WI signal intensity | Intermediate | Bright |
| Tympanic membrane retracted | Yes | No |
| Tegmen tympani erosion | Sometimes | No |
| Facial nerve canal dehiscence | Sometimes | No |
Complications of cholesteatomas include fistulization into the semicircular canals (from 4% to 25% of cases), with the lateral semicircular canal most commonly affected. This may be identified as a dehiscence in the bony labyrinth with a soft-tissue mass expanding the region of the oval window or lateral margin of the lateral semicircular canal. Alternatively, cholesteatomas may erode the tegmen tympani (the roof of the epitympanic space) and subsequently invade the intracranial compartment (Fig. 12-19). Another area of potential erosion is the lateral or inferior wall of the tympanic portion of the facial nerve (Fig. 12-20). If there is dehiscence or skeletization of the facial nerve canal or the sinus tympani, the surgeon must know this preoperatively so that removal of the cholesteatoma is done in a careful fashion so as not to injure the underlying structures.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree