CHAPTER 41 Tentorial and Falcotentorial Meningiomas
INTRODUCTION
Meningiomas of the posterior cranial fossa account for about 9% of all intracranial meningiomas.1 Approximately 30% of all posterior fossa meningiomas arise from the tentorium cerebelli.2 The first account of a tentorial meningioma was given by Antral in 1833 as an incidental finding.3 Meningiomas of the falcotentorial junction are considered a subgroup of tentorial meningiomas and constitute about 0.3% to 1.1% of all intracranial meningiomas.4–7 Surgical morbidity and mortality after resection of tentorial/falcotentorial meningiomas have declined steadily as a result of refinements in microsurgical and neuroanesthetic techniques and improved pre- and postoperative patient care. Nonetheless, resection of these tumors remains a major surgical challenge due to their proximity to the brain stem and frequent involvement of critical neurovascular structures, particularly major venous sinuses and the deep venous system.
TENTORIAL MENINGIOMAS
Classification
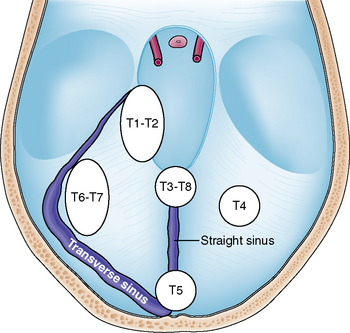
Tumors arising from the tentorium may be confined to the supratentorial or infratentorial compartment or may extend both infra- and supratentorially. Several classification schemes have been proposed for these tumors.8–11 Yaşargil introduced the most accurate of these classifications in regard to surgical anatomy and differentiated among the following tumor subgroups:11 (1) meningiomas arising from the free tentorial notch (i.e., inner ring meningiomas, anterior T1, middle T2, and posterior T3); (2) meningiomas originating from the intermediate tentorial surface (T4); (3) meningiomas involving the torcular herophili (T5); (4) meningiomas arising from the lateral outer tentorial ring (posterior T6, anterior T7); and (5) falcotentorial meningiomas (T8). In our retrospective study of 81 patients harboring a tentorial meningioma and who were treated microsurgically it was usually not possible to accurately differentiate between T1 and T2 and T6 and T7 tumors from preoperative radiologic studies.4 Further, T3 and T8 tumors originate from both the falx and tentorium. Therefore, we consider them, by definition, as falcotentorial meningiomas. For these reasons and for practical purposes, we prefer a modified Yaşargil classification scheme comprising the following tumor subgroups (Fig. 41-1):
Clinical Presentation
Clinical symptoms and signs depend on location and size of the tumor. Patients harboring a lateral tentorium meningioma (T6–T7 subgroup) usually present with headache, dizziness, and gait unsteadiness.4,12 Clinical examination often reveals a gait ataxia and involvement of the VIIIth cranial nerve (CN).4 It may be difficult to differentiate between lateral tentorial and suprameatal posterior petrosal meningiomas on clinical and radiologic grounds.13 The exact origin of the tumor may be recovered only during surgery. Tentorial notch meningiomas (T1–T2 subgroup) usually intimately involve the brain stem. Accordingly, these patients may present with a hemiparesis and impairment of the Vth CN, namely trigeminal neuralgia or facial numbness.14,15 Patients with large tumors may complain of symptoms of raised intracranial pressure due to the accompanying hydrocephalus. Presentation with epileptic fits points to a supratentorial meningioma, which may be in proximity to the medial temporal lobe. Apart from direct involvement of the VIIIth CN, preoperative hearing impairment may also be caused by distortion of central auditory pathways, such as the lateral lemniscus or inferior colliculi.4,16–18 In either case, hearing impairment often shows remarkable improvement after surgery.4,17
Preoperative Considerations and Diagnostic Workup
Elderly patients presenting with an incidental finding of a small tentorial meningioma who are asymptomatic or have minor unspecific complaints such as headache or dizziness may best be observed with serial clinical and radiologic follow-up. If the patient becomes symptomatic and/or the tumor is progressive in size, surgical removal may be warranted. Alternatively, radiosurgery may be more appropriate in selected cases, particularly in older patients who are not good surgical candidates due to significant co-morbidity. When surgery is considered, magnetic resonance imaging (MRI) with multiplanar images provides the most valuable preoperative diagnostic tool. Computed tomography (CT) scanning, although depicting tumor calcification in some cases, is of minor importance because of its inherent inaccuracy to demonstrate tumors in the posterior cranial fossa and because bone involvement is not a prominent feature in tentorial meningiomas. MRI accurately delineates the location and extent of the tumor. Further, the relationship of the tumor to the basilar artery, for example, displacement or encasement, is demonstrated. Venous sinus patency and the drainage pattern of the Labbé venous complex into the transverse sinus can be nicely depicted from MR venograms (MRV). This information is of particular importance if subtemporal or transpetrous approaches are being considered. Preoperative MR angiography has largely replaced invasive catheter angiography, particularly as embolization of meningiomas in this location is not considered a useful preoperative adjunct.4,18 Hyperintensity of the brain stem adjacent to the tumor on T2-weighted images may point to disrupture of the arachnoid barrier layer and difficulties in microsurgical dissection of the tumor from the brain stem may be anticipated. In our experience, even the most sophisticated preoperative neuroimaging tools available today have important shortcomings. It is usually not possible to exactly define the relationship of the tumor to cranial nerves (integrity, site of displacement, infiltration). Tentorial sinuses usually cannot be visualized on preoperative imaging and may be a source of significant intraoperative bleeding in transtentorial approaches.19 The degree of infiltration of the venous sinus by tumor can be fully appreciated only during surgery. A nonpatent venous sinus on preoperative angiographic studies (MRV or catheter angiography) may prove to be patent during surgery. Finally, information on the functional significance of major venous sinuses or veins is usually lacking.
Surgical Approach and Technique
The primary goal of treatment is complete microsurgical removal of the tumor, including resection of its dural matrix. This is the best means to prevent a tumor recurrence. Of equal importance is preservation of integrity of neurovascular structures and function. The surgical approach should be tailored according to the tumor site and extension (Table 41-1) and should be planned after careful analysis of preoperative radiologic images. Supratentorial tumors of the anterior inner and outer ring, that is, supratentorial T1–T2 and T6–T7 meningiomas, are usually best approached via a subtemporal route. Positioning of the patient’s head to take advantage of gravity and preoperative placement of a lumbar drainage may facilitate temporal lobe retraction. Retraction of the temporal lobe jeopardizes the subtemporal and Labbé venous complex with the potential of major neurologic sequel. Adding a zygomatic osteotomy provides a flatter angle of view to the lesion, resulting in a lesser degree of temporal lobe retraction, but does not completely abandon the risk to the venous system.9,20 Sugita and colleagues have emphasized that preservation of subtemporal veins is facilitated by a wide temporal craniotomy, particularly in horizontal length.10 The venous anatomy should be studied carefully on the preoperative MRV with special attention paid to the drainage site of the temporal veins into the transverse sinus.21 In patients without tumor extension to the upper surface of the petrous bone, a paramedian supracerebellar transtentorial approach has been successfully applied to resect medial tentorial meningioma and avoid the veins of the temporal lobe.22
TABLE 41-1 Location of tentorial meningioma, recommended surgical approaches, and associated potential complications
| Tumor location | Surgical approach | Potential complications* |
|---|---|---|
| T4 (paramedian) Infratentorial | Supracerebellar infratentorial | Gait ataxia |
| T5 (peritorcular) Supra–infratentorial | Gait ataxia | |
Modified from Yaşargil’s classification.11
The conventional retrosigmoid approach is suitable for most infratentorial meningiomas of the inner and outer ring (infratentorial T1–T2 and T6–T7 tumors). Alternatively, the supracerebellar infratentorial route via a suboccipital craniotomy may be more appropriate in some more medialized infratentorial T1–T2 tumors. A supratentorial tumor extension in these cases can be removed transtentorially. The supracerebellar infratentorial avenue is the approach of choice in infratentorial paramedian T4 meningiomas. In our experience it was usually sufficient to resect the tumor along with its attachment on the outer dural layer to achieve a Simpson grade 1 resection.4,23 Supratentorial T4 tumors are resected via an occipital approach. Again, a smaller infratentorial tumor portion can be resected transtentorially.24
Complications and Outcome
Surgical mortality and morbidity has steadily declined over the years. Recent microsurgical series report a mortality of 2.5%,4 3.7%,16 0%,14 and 2.7%.12 Surgical morbidity has ranged between 19% and 55% in recent microsurgical series.4,12,14,16 However, most complications were transient and resolved on follow-up.4,16 Postoperative CN morbidity is related to the tumor site and to the approach selected (see Table 41-1). CNs III, IV, and V are endangered during resection of T1–T2 tumors and in the subtemporal and transpetrous approach while CNs VII and VIII are jeopardized in T6–T7 tumors and the retromastoid approach.4 To reduce the incidence of postoperative complications, tumor resection should strictly follow the arachnoid cleavage plane. However, the arachnoid may be disrupted in larger tumors. In these cases, it is preferable to leave a tuft of tumor on critical structures, namely the brain stem, cranial nerves, and blood vessels, to prevent serious neurologic sequel or even surgical fatalities.4
A total tumor removal, defined as a Simpson grade 1 and 2 resection, is reported in 77% to 91% of the patients.4,8,12,14,16 A complete tumor resection is usually achieved in T4 meningiomas, while a total removal is least often feasible in T1–T2 and T5 tumors. A tumor recurrence or progression after subtotal removal is observed in 0% to 26%4,8,12,14–16 in larger series with a long follow-up. A major issue of controversy is whether to resect an infiltrated dural venous sinus to achieve a complete tumor resection and thus decrease the tumor recurrence rate. There is general agreement that a completely occluded sinus can be resected safely.16,25 However, it needs to be remembered that parts of the deep venous system, including venous sinuses, may not be visible on preoperative angiograms, although they are neither occluded nor functionally unimportant.26 In addition, angiograms are prone to underestimate sinus infiltration by tumor.4,8,15 Atresia or septation in the region of the sinus confluence may lead to misinterpretation with regard to dominance of the venous sinus.27 Current data in the literature do not demonstrate a lower tumor recurrence rate in series in which aggressive resection of involved venous sinuses was performed.8,12,15 Further, it has been demonstrated that subtotal removal of meningioma can be associated with a long-progression-free period and high quality of life.28 We therefore recommend preservation of an infiltrated but patent dural venous sinus and have performed either resection of the infiltrated outer dural layer of the sinus or careful coagulation of residual tumor on the sinus wall with low bipolar current. Stereotactic radiosurgery may be a useful treatment option for patients with residual or recurrent tentorial meningiomas. A recent small series using stereotactic radiosurgery in recurrent or incidental tentorial meningiomas reported an overall tumor control rate of 98% after a mean follow-up period of 3 years.29
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree