Fig. 20.1
Prism adaptation. Top: the three steps of prism adaptation procedure. (1) Baseline alignment of vision and proprioception is measured by an open-loop pointing task (i.e. pointing to a visual target without visual feedback). (2) The introduction of prisms shifts movements to the right of the visual target. (3) When visual feedback is allowed subjects compensate for this visuomotor misalignment by shifting proprioception in the direction opposite to the optical shift and vision in the shifted direction, so that they gradually resolve the misalignment. (4) Realignment is quantified by measuring the pointing bias after removal of the prisms in comparison to the pretest (redrawn from Rossetti et al. [21]). Bottom: another measure of prism after-effect is obtained by asking participants to point straight ahead of their body midline in the dark. This egocentric reference demonstration is typically shifted in neglect patients (pretest) and improved following prism adaptation (post-test). This proprioceptive component of adaptation (effect) compensates the optical deviation by shifting proprioceptive representations in the direction opposite to the prismatic shift (redrawn from Rossetti et al. [1])
In the following sections we will see that similar families of compensation can be proposed following a brain lesion: top-down approaches based on descending control processes and bottom-up approaches based physiological sensorimotor manipulations.
Following exposure to a rightward optical deviation of the visual field, subjects show a systematic leftward deviation of visuomotor and proprioceptive responses with the adapted limb (see Fig. 20.1). The crucial observation is that these sensory and motor biases are opposite to those described in UN patients without adaptation [26]. Our original proposal [1] was to use these PA-induced sensorimotor alterations as a method to reorient neglect patients’ behaviour towards the neglected side and tentatively amend their deficits. Another interesting feature of the sensorimotor reorganisation induced by PA is that it can be produced without requiring patients’ voluntary attention to their left-sided deficit, i.e. according to a bottom-up process that bypasses their damaged awareness and intentional control (see Rode et al. [3]).
20.2.1 Spatial Neglect: Cognitive and Sensorimotor Symptoms
Spatial neglect is regarded as complex neurological syndrome, which is defined as the patient’s failure to report, respond to, or orient towards novel and/or meaningful stimuli presented to the side opposite of the brain lesion [27–29]. This condition is frequently found in brain-damaged patients, often in association with contralesional hemiplegia, hemianaesthesia, and hemianopia. Neglect thus constitutes a space-oriented behavioural disorder with an ipsilesional bias, typically towards the right side. The subject with neglect spontaneously displays a tonic ocular and cephalic deviation towards the right side. This behavioural bias will also be evidenced in many clinical tests, such as in cancelling lines, searching for an object, or pointing to a straight-ahead position in darkness [30]. The core phenomenon of neglect is that this behavioural ipsilesional bias is associated with unawareness of contralesional space [31–33]. The subject with neglect is thus unable to compensate his or her illness by a voluntary orientation of attention—a situation quite unlike the patient with hemianopia who can nevertheless orient his or her gaze towards the blind hemi-field.
Neglect is mostly found following a lesion of the right hemisphere which is responsible for a deficit for the left side. The deficit observed in spatial neglect applies both to body space and extra-personal space. Neglect patients typically exhibit inattention to sensory stimuli, and several aspects of their body schema appear to be impaired as well, i.e. at the sensorimotor level. External neglect is manifested for stimuli delivered in any sensory modality, although it has been mainly studied in the visual domain. Patients just appear to be omitting the items that are presented to their left. They may present with sustained eye and head deviations to the right or estimate the straight-ahead direction to be shifted to the right. They may produce slower movements to the left and even progressively omit to move the left hand during bimanual taping. Their visuomotor use of space can also be severely impaired (Fig. 20.2). At the cognitive level, in addition to the well-known and striking visual symptoms, their body image can be affected as well. They may show anosognosia, i.e. the lack of awareness for a left-sided deficit such as hemiplegia, or even somatoparaphrenia, i.e. delusion about their own body, especially about their neglected side. Personal neglect may range from an impoverishment of the representation of one side of the body to a distortion of the representation of the whole body. Higher-order alterations of body image are exhibited by some neglect patients, for example, in the form of drawing only one half of their self-portrait. These higher-order deficits contrast with the preserved visuomotor abilities and are compatible with the view that neglect is primarily a deficit of conscious access and use of spatial information. One of the classical, though still being debated, symptoms of spatial neglect is a leftward shift of the manual demonstration of the subjective straight ahead (Fig. 20.1). This apparently basic bias is usually interpreted as a misrepresentation of the egocentric reference and again can be contrasted with the good visuomotor performances of neglect patients (Rode et al. [86]). This argument is of prime importance as it has been argued that the egocentric reference assessed by manual pointing “straight ahead” provides the reference axis for the organisation of interactions with the external world (for a review, cf. [26]). Building up on this pathophysiological hypothesis, prism-adaptation-induced manipulations of sensorimotor interactions have been studied in patients with spatial neglect. As we have discussed above, a behavioural bias may be experimentally induced by a simple prism adaptation procedure [8, 26, 35]. The relevant point for unilateral neglect rehabilitation is that after a rightward optical deviation of the visual field, subjects show a systematic leftward deviation of visuomotor and proprioceptive responses with the adapted limb. We proposed to use this after-effect as method to help neglect patients reorient their behaviour towards the neglected side (Fig. 20.1). The advantage of this reorientation is such that it may be produced without requiring the patient’s voluntary attention, i.e. according to a bottom-up process that bypasses awareness and intentional control which are both affected by neglect. The next two sections will be devoted to these results and their clinical implications.
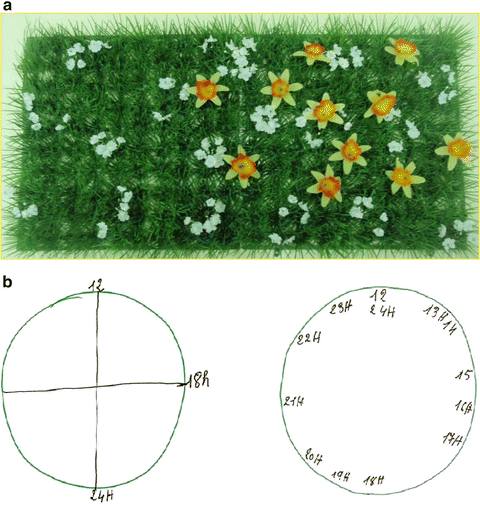

Fig. 20.2
Unilateral neglect. Top: the gardening test. The patient is given ten daffodils and asked to distribute them homogeneously in the green patch of grass scattered with tiny white flowers. This test evaluates the ability of the patient to invest the available space instead of search for targets as in cancellation tests. It is easily quantified by recording the location of each daffodil. Here the patient obviously planted flowers only in about one half of the garden whereas he was able to cancel about ¾ of the bell test. This simple test can be easily quantified with several measures: average position of the 10 flowers along the horizontal axis (depicting the mean planting bias), variability of this horizontal position (depicting the horizontal span covered by the patient), and the distance between the left edge and the left-most flower (depicting the maximal orientation to the left). Bottom: two drawings of clock face. The left drawing illustrates spatial neglect for the left half of the clock. The right drawing illustrates neglect for small numbers, whereby patients having set the main landmark on the face (i.e. 12) are unable to move backward (i.e. to the left) in the number space and to activate a representation of “1”. They instead move towards larger number (i.e. to the right) and write “13” in the place of “1” and proceed up to “24”. The left drawing also combines this number neglect to the obvious spatial neglect (redrawn from Rossetti et al. [34])
20.2.2 Prism Adaptation in Neglect: Sensorimotor Adaptation Without Cognitive Contribution
Two approaches may be distinguished in the rehabilitation of neglect: a “top-down” and a “bottom-up” lines of attack [3, 5]. The first approach is a pragmatic clinical approach which is aimed at improving the perceptual and behavioural biases by acting on the patient’s awareness of the deficit, i.e. at the highest cognitive levels, including training in visual scanning, cueing, or sustained attention [36, 37]. The second approach is based on physiological grounds and is aimed at modifying the sensorimotor level by passive sensory manipulations, visual deprivation or by visuomotor adaptation which bypasses the central awareness deficit and indirectly influences the highest levels of space and action representation [2, 3, 38–40]. Numerous manifestations of neglect have been shown to be alleviated by sensory stimulation (vestibular, optokinetic, transcutaneous electrical, transcutaneous mechanical vibratory and auditory). Prism adaptation is currently viewed as the most studied technique and is considered as the most promising [41].
In the first prism rehabilitation study, we [1] demonstrated that a short period of pointing (50 movements over 2–5 min) towards targets viewed under a 10° rightward displacement, resulted in a shift in manual straight-ahead pointing towards the left side (after exposure, the neglect patients produced an average pointing bias of just 2° rightward, i.e. showing an after-effect of about 7° (or 80 % compensation for the 10° optical displacement)). This shift of proprioceptive representations towards the neglected side was associated with a reduction of the rightward bias observed in visuo-manual tasks, such as line cancellation, line bisection, and drawing a daisy from memory. Since this study, numerous papers have been published about the effects of PA on symptoms of neglect (review in: Jacquin-Courtois et al. [42]; see video tutorial at: http://www.chu-lyon.fr/web/4531).
Using prism adaptation in neglect patients rapidly led to the observation that they do not exhibit emotional reactions when producing their first pointing movement with prisms on, whereas other participants, e.g. their partners, inevitably do markedly react to the first trial misreach. We therefore systematically investigated patients’ awareness for the optical shift through a specifically designed questionnaire. This questionnaire comprised 20 items starting with very open questions and ending with accurately targeted questions (Table 20.1), in order to properly assess the level of implicit to explicit knowledge about the behavioural consequences and the nature of the prism-induced visual distortion. Strikingly straightforward results emerged from this study. None of the 20 patients included in this study exhibited the slightest sign of explicit or implicit knowledge that anything unusual happened during the pointing session, despite being very explicitly asked about possible effects on movement guidance. Further testing with this questionnaire over the last ten years revealed that the only two neglect patients who provided evidence for some awareness for the optical shift had been submitted to several rehabilitative prism adaptation sessions. This result may be interpreted in several ways. First, it can result from the implicit knowledge of the patients that their behaviour has lost accuracy. This may be described as hypernosognosia, as if the patient attributed more errors to their own disorder than appropriate. Second, it may be that areas responsible for the awareness of such pointing errors are associated with neglect anatomical networks. Third, the patients’ lesion may interfere with brain functions involved in the strategic compensation of the optical shift. The first hypothesis would require support from further demonstrations that such self-attribution of error may occur during other tasks. The second and third possibilities are theoretically valid although they may appear incompatible by the wide variety of brain lesions leading to unilateral neglect. It remains nevertheless striking that patients remain totally unaware of the optical manipulation produced by prisms whereas they are able to correct their pointing movement on flight in order to produce negligible terminal errors.
Table 20.1
Prism awareness questionnaire
1. After 10 pointing movements with prismatic glasses off : |
Q1. How is this exercise going? |
Comment se passe l’exercice? |
Q2. Isn’t it not too complicated? |
Est-ce que ça n’est pas trop difficile? |
Q3. Have you observed anything in particular? |
Est-ce que vous avez observé quelque chose de particulier? |
Q4. Do you see the target well? |
Est-ce que vous voyez bien la cible? |
Q5. Is it easy to aim towards the target? |
Est-ce que c’est facile de viser vers la cible? |
2. After 5 pointing movements with prismatic glasses on (exposure): |
Q6. How is this exercise doing? |
Comment se passe l’exercice? |
Q7. Isn’t it too complicated? |
Est-ce que ça n’est pas trop difficile? |
Q8. Have you observed anything in particular? |
Est-ce que vous avez observé quelque chose de particulier? |
Q9. Do you see the target well? |
Est-ce que vous voyez bien la cible? |
Q10. Is it easy to aim towards the target? |
Est-ce que c’est facile de viser vers la cible? |
Q11. Do you find the glasses special? |
Les lunettes vous semblent-elles spéciales? |
Q12. Is it easier or more difficult to point with the glasses on? |
Est-ce plus facile ou plus difficile de pointer avec les lunettes? |
Q13. Do you have the impression that you’re making pointing errors? |
Avez-vous l’impression de faire des erreurs de pointage? |
Q14. Do you have a tendency to aim more on one side than on the other? |
Avez-vous tendance à viser plus d’un côté que de l’autre? |
Q15. Do you have the impression that your hand is drawn more towards the right or towards the left? |
Avez-vous l’impression que votre main est-elle attirée vers la droite ou vers la gauche? |
Q16. In some patients these glasses occasionally cause sight problems. How about you? |
Chez certains patients ces lunettes posent parfois des problèmes de vue. Comment cela se passe-t-il pour vous? |
Q17. In some patients these glasses can render it difficult to aim with the hand. How about you? |
Chez certains patients ces lunettes peuvent entraîner des difficultés pour viser avec la main. Comment cela se passe-t-il pour vous? |
3. After 20 pointing movements with prismatic glasses on (exposure): |
Q18. Do you have anything to say about this exercise? |
Avez-vous des remarques à faire au sujet de cet exercice? |
4. At the end of prism exposure: |
Q19. Do you have anything to say about this exercise? |
Avez-vous des remarques à faire au sujet de cet exercice? |
Q20. In some patients these glasses can render it difficult to aim with the hand. How about you? |
Chez certains patients ces lunettes peuvent entraîner des difficultés pour viser avec la main. Comment cela se passe-t-il pour vous? |
A further investigation attempted to seek confirmation for this apparent lack of awareness by measuring the emotional responses of patients. It is important to bear in mind that healthy individuals exposed to prisms inevitably and most spontaneously exhibit explicit emotional reactions. Given that no such explicit reaction was observed in patients we investigated their galvanic skin response (GSR) in comparison to controls. As shown in Fig. 20.3, subjects were seated in front of a wheel fitted with prisms that produced either zero, 2° or 8° of visual shift. During the simple visual pointing task the wheel was rotated between each trial even if most trials involved 0° shifts. In rare cases the 2° or 8° visual shift was introduced unbeknownst to the subjects who had to keep their eyes closed except just before and during the pointing and then could only see a pointing target over a homogeneous background. The pointing target was a laser dot projected at a random position in front of them in order to not provide the subject with any information about the presence of a visual shift prior to seeing their hand movement deviated. All subjects transiently showed some degree of GSR following each pointing movement. Healthy subjects systematically exhibited a significantly stronger skin conductance increase when the 8° shift was introduced, but not the undetected 2° prism, showing the link between awareness of the shift and GSR intensity. When patients were tested, neglect was associated with the disappearance of the normal GSR, which further confirms converging information about their lack of awareness for the optical shift induced by prisms.
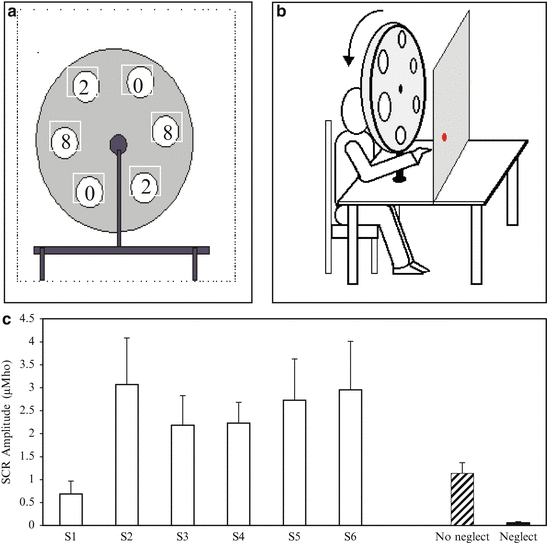

Fig. 20.3
Skin conductance during prism adaptation. Top left: the wheel fitted with 0° (sham), 2° (control) or 8° (test) deviating prisms. Each prism was present twice in order to allow wheel rotation even when successive trials involved a 0° shift. Top right: the subject was seated in such a way that the right eye sees the homogeneous pointing plane through the prism window and the left eye sight is blocked. Bottom: Skin conductance was continuously monitored and inter-trial intervals lasted at least 10 s. There was a significant increase of the skin response to the unpredictable introduction of the 8° shift in healthy subjects (S1–S6) and in the control (No neglect) but not in the neglect patient (Neglect). No such increase was observed following the 2° shift introduction that remained undetected in all participants
There are several potential consequences of suppressing awareness for the optical shift. First it may be that subjects adapt more because this lack of awareness prevents the brain from using cognitive compensations for the optical shift, which may result in an increase of true sensorimotor adaptation. These cognitive compensations have been shown (e.g. [11, 20]) to effectively reduce direct effects of prisms (i.e. pointing errors during prism exposure) while not resulting in after-effects (i.e. open-loop pointing errors at the prism removal). And the lack of awareness for the optical manipulation that results from a gradual introduction of the visual shift was shown to increase the amount of after-effects in healthy individuals [43]. Second, it may be that the lack of awareness alters the ratio of visual and proprioceptive after-effects. This was suggested by Rossetti et al. [1] on the basis of larger proprioceptive after-effects in neglect patients and challenged by Sarri et al. [44] who measured both the proprioceptive and the total after-effects in these patients (see also Rode et al. [86]). Third, it seems reasonable to argue that patients go through deeper levels of sensorimotor adaptation because the duration of their after-effects is much longer than expected in healthy subjects (e.g. [25, 45]). At least one study found a significant correlation between improvement of visual neglect assessed by a cancellation task and the amount of the after-effect subjective assessed by subjective straight-ahead pointing [44]. We will now discuss the surprising expansion [42] of prism adaptation in cognitive domains that appear independent from the visual and motor components activated during prism exposure.
20.3 Sensorimotor Adaptation in Neglect: Cognitive Rehabilitation
In most early studies of prism adaptation in neglect, the main tests used to assess the effects of prism training required a visuo-manual response, that is, the use of a physiological system that is directly involved in the adaptation procedure. Repeated evidence has been provided that the beneficial effects of prism training are not restricted merely to visuomotor tasks, but can also be described for non-motor and non-visual tasks (see review in Jacquin-Courtois et al. [42]).
Several visuo-verbal (i.e. non-manual) tasks used to test the effects of PA include objects or room description, letters, words, sentences or texts reading [1, 45–50]. Temporal order judgement tasks [51], size comparison tasks [52] and perceptual awareness [53] have also been improved following prism adaptation. Other non-visual functions positively affected by prism adaptation come from the mental representation domain, free from manual responses and overt visual scanning. Representational neglect was explored in two tasks: explicitly spatial (the map of France [24, 54]) and not-explicitly spatial (mental number bisection, [55]). On both tasks, the pretest rightward bias was largely improved after a single session of PA, showing that PA can reduce representational neglect. This result is important because it strongly demonstrates that cognitive effects of prism adaptation are highly independent from the trained visuomotor task.
Vision is the sense being manipulated through prism exposure, and it is crucial that the expansion of prism adaptation is explored in other sensory modalities. The somatosensory modality is directly affected by prism adaptation (Fig. 20.1) in terms of coding arm position in space. Typical after-effects result in shifting the internal representation of the extended arm by a few degrees. However other beneficial effects have been described in unexpected somatosensory domains. McIntosh et al. [48] assessed effect of PA on a haptic spatial judgement task in a single chronic neglect patient. In this task, with no visual information, the patient had to locate the centre of a haptically explored circle. The patient was examined in four sessions, spaced at weekly intervals; PA was performed during the three last sessions. Results showed not only improvement of symptoms of neglect at a chronic stage but also positive results on this circle centring task with no visual component. In a more recent work (Jacquin-Courtois et al., in preparation), we explored the effect of PA on a haptic discrimination task in three neglect patients. Patients were blindfolded and required to explore two objects with the right hand. Then they simply had to verbally report whether the objects were identical or not (i.e. “same” or “different”). The shapes of these objects were either symmetrical or not, and the difference between two objects could be on either the left or the right side. The improvement obtained following PA primarily affected the left-sided errors, but was also observed for right-sided errors. These results demonstrate that neglect can affect haptic discrimination and that PA can improve non-visual shape processing in a non-exposed modality.
In the tactile domain, Maravita et al. [56] showed an improvement of contralesional tactile extinction in four neglect patients. Tactile stimuli were delivered unilaterally either to the right, the left, or to both hands simultaneously. Detection of contralesional tactile stimuli during bilateral stimulation improved after PA in all patients. More surprisingly, tactile detection thresholds and proprioceptive perception also improved in a single case study [57].
Beyond the somatosensory domain, Jacquin-Courtois et al. [58] assessed the effects of PA on auditory extinction, an auditory deficit frequently occurring in right brain-damaged patients with neglect. This deficit, whose clinical manifestations are independent of the sensory modalities engaged in visuo-manual adaptation, was quantified in neglect patients before and after PA, by means of a verbal dichotic listening task (Fig. 20.4). The results demonstrated that PA can improve left auditory extinction, thus revealing transfer of benefit to a sensory modality that is independent from the visual modality involved in adaptation. The observed benefit was specific to the detection asymmetry between the two ears and did not affect the total number of responses. This shows that the effect of PA applies to lateralised processes rather than on general arousal. Whereas somatosensory effects could have benefited from the direct effect of prism adaptation on some aspects of proprioceptive coding, this auditory benefit provided strong support to the idea that PA effects expand to unexposed sensory systems.
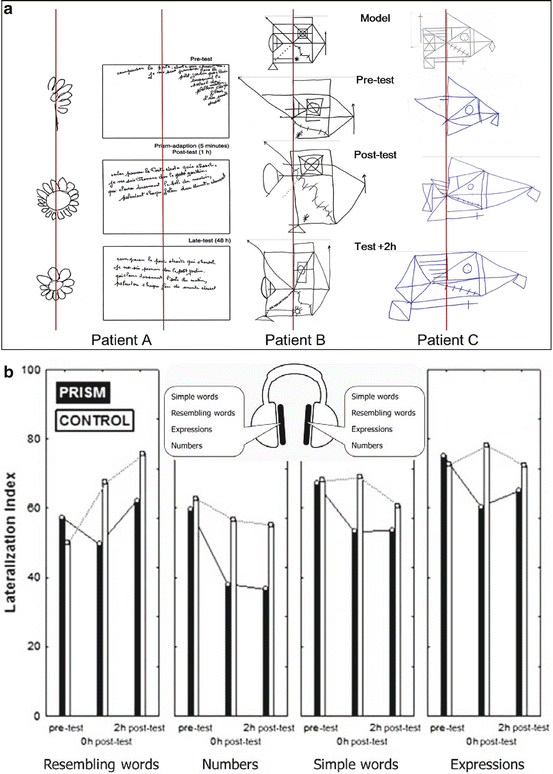

Fig. 20.4
Cognitive effects of prism adaptation. Top: daisy drawing, dictation and complex figure copying by three neglect patients before (pretest), immediately after (post-test) and 2 h after (test +2 h) prism adaptation. The patients exhibit improvement not only for the spatial component of neglect but also for the constructional aspect of the writing lines and figure copying (redrawn from Rode et al. [5, 59]). Bottom: dichotic listening in neglect following sham (control) or real prism (prism) pointing exposure. Patients were tested before, immediately after or 2 h after prism adaptation with list of words presented simultaneously to the two ears. Values of the lateralisation index above 50 reflect the classical neglect bias towards the right, i.e. omission of words presented to the left ear. This index was systematically reduced after prisms and the beneficial effect tended to last for at least 3 h (redrawn from Jacquin-Courtois et al. [58])
Another interesting category of tasks may have been affected by prism adaptation by a more complex two-step interaction between sensorimotor and cognitive levels. These tasks are acknowledged to involve very automatic levels of control, are not exposed to adaptation during prism exposure and remain largely independent from cognitive control in everyday life. Therefore they do not appear to be directly affected by a simple bottom-up effect of adaptation but rather by a reverberation of this effect down onto the concerned sensorimotor levels of control. The first example of such two-way interaction effect is oculo-motricity. Several studies have reported an improvement of oculo-motor patterns after PA (more leftward exploratory movements, reduction of mean right-shift fixation position). This improvement may not be accompanied by improvement of contralesional space unawareness in tasks such as size underestimation, judgement of vertically arranged pairs of chimeric faces or free visual exploration task [52, 60, 61]. It is worth noting that an important heterogeneity of oculo-motor exploratory patterns has been observed in neglect patients, presumably due to association of multiple variable components according patients. Shiraishi et al. [62] studied long-term effect of PA in chronic neglect patients using daily sessions of game-like activities instead of the usual visual pointing task. Eye movements were significantly improved on the neglected side after the repeated intervention, and the effects were sustained for up to 6 weeks after end of the treatment. These results suggest that oculo-motor parameters can be significantly improved by PA. Whether this effect can be ascribed to low level sensorimotor effects of adaptation or to higher level consequences of adaptation is difficult to disentangle.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







