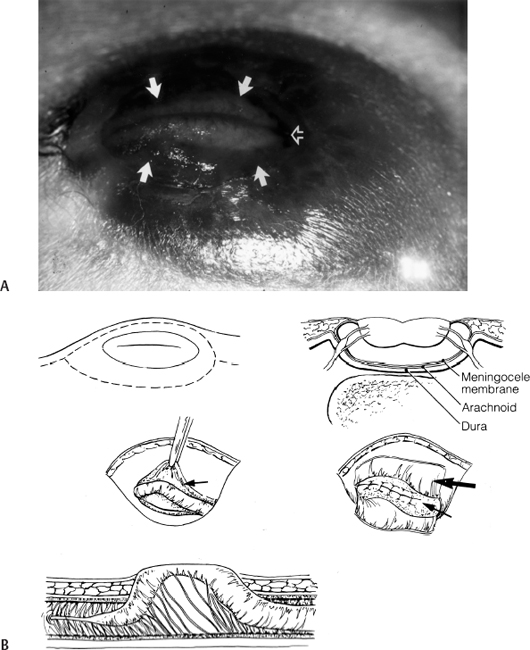
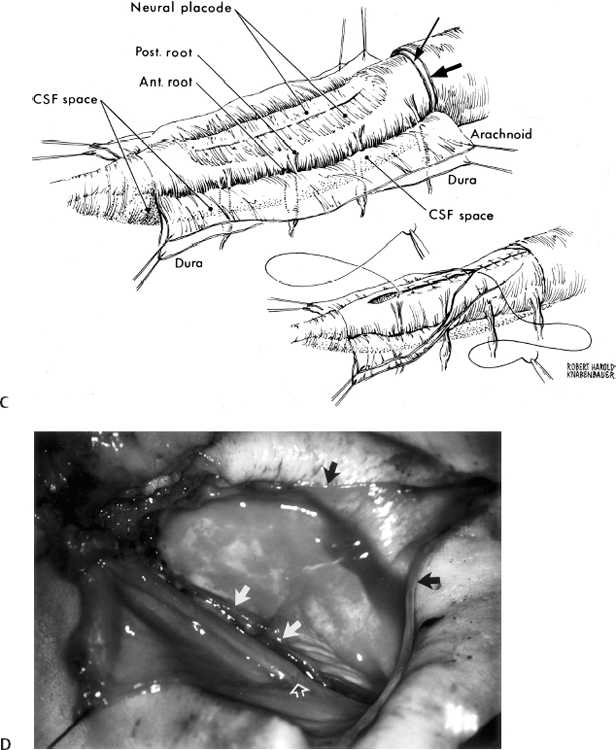
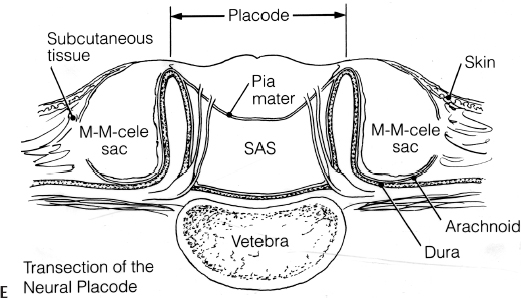
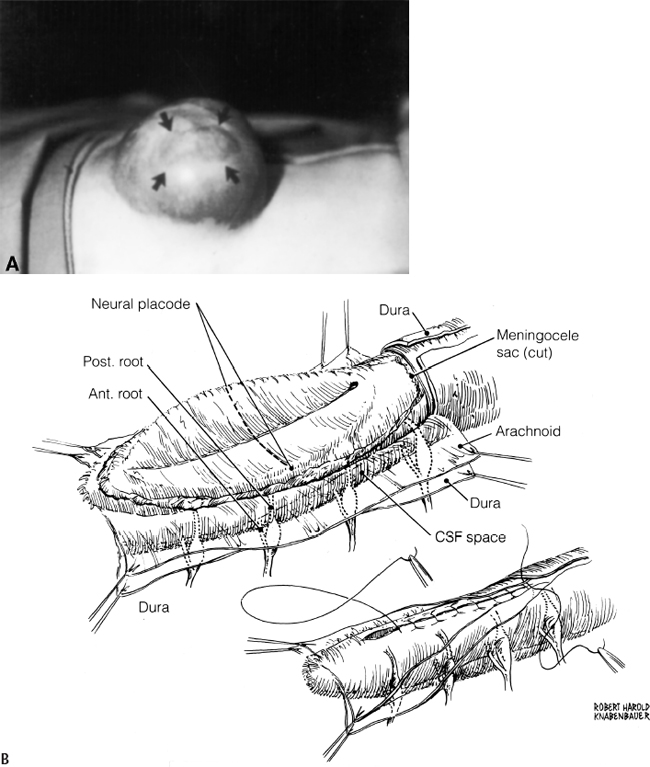
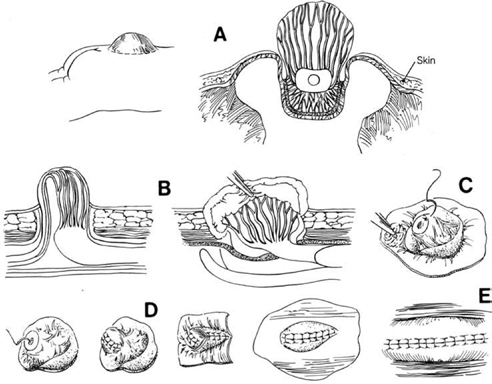
11 Tethered Cord Syndrome Associated with Myelomeningoceles and Lipomyelomeningoceles Shokei Yamada, Daniel J. Won, Alexander Zouros, and Shoko M. Yamada This chapter concentrates on tethered cord syndrome (TCS) associated with two pathological categories: (1) myelomeningoceles (MMCs) and (2) lipomas or lipomyelomeningoceles (LMMCs). Earlier the goal of surgical treatment for MMCs, spina bifida aperta, was considered to be protection of the neural elements and prevention of infection to the exposed spinal cord and intradural-subarachnoid space,1,2 and the consequent scar formation. It became apparent, however, that repair of MMCs in some patients, and of lipoma and LMMCs in the majority of patients, was followed by neurological improvement. Such experiences were supported by research work with the following evidence. The impaired oxidative metabolism was associated with increased tension in the lumbosacral cord, and further, metabolic improvement occurred after release of tension by the repair of these anomalies.3 It is a reasonable assumption that these clinically or radiologically demonstrable anomalies must be eliminated surgically if the signs and symptoms suggest the presence of TCS. Furthermore, untethering procedures should be performed on symptomatic patients at an early stage if operative risks are estimated as minimal. This chapter focuses on the symptomatology, prevention, and treatment of TCS. MMCs in the lower lumbar and sacral regions are often associated with TCS. Until recently, it was believed that neurological deficits associated with MMCs were neither reversible nor progressive.4,5 Thus it was assumed that the repair of such lesions was purely for better nursing care and cosmetic reasons. Understanding of the pathophysiology of TCS and the delayed neurological deterioration observed in children with spinal cord tethering has brought to light the importance of early correction and avoidance of retethering through proper surgical repair.6,7 In general, MMCs are the result of defective neurulation. The normal tubular formations of the dura mater and pia arachnoid remain cephalic and caudal to the dysraphic level. The dura is usually open and everted over the articular facets and often extends farther laterally to cover the paraspinal fascia. The arachnoid membrane is frequently everted and covers the inner side of the dura. However, when the normal skin covers the major portion of the MMC, the arachnoid usually underlies the skin and extends over the membranous sac. The arachnoid membrane can be clearly isolated and closed over the neural tissue. The pia mater is widely open lateral to the neural plaque, and the central canal terminates at the cephalic end of the neural placode and continues as a midline groove of the placode. The MMC sac itself is composed of an aberrant membrane or an extension of the intermediate arachnoid,8 which is interposed between the pia arachnoid and pia mater in the normal spinal canal. Hereafter, the MMC sac is referred to as the meningocele or meningeal sac. Nerve roots often pass through a layer of the meningocele sac or internal to the membrane. Cerebrospinal fluid (CSF) contained in the MMC communicates with the normal subarachnoid space at the junction of the meningocele sac and normal spinal canal. CSF probably circulates through the numerous small channels between the arachnoid trabeculations or the penetrations of the intermediate arachnoid formed at the base of the MMC. The dispute has been long-standing as to whether early surgical intervention is beneficial in cases of MMC. Previously, no neurological improvement was expected after the repair of MMCs.4,5 However, an increasing number of cases with postoperative neurological improvement have recently been reported,6,7,9 and these results have encouraged early surgical intervention. It is now generally accepted that repair and closure of MMCs should be performed within 48 hours of birth.4–6 It is believed that the repair of MMCs associated with untethering of the spinal cord may ameliorate neurological deficits,6,7 particularly motor and sensory function.6 The repair and closure of MMCs have the following objectives: (1) to protect the externally exposed neural elements; (2) to prevent infection caused by the invasion of organisms; (3) to prevent and reverse neurological deficits by untethering the spinal cord6,7; (4) to prevent retethering after surgery6,7,10; (5) to improve nursing and, later, self-care; and (6) to facilitate cosmetic reconstruction. Prior to surgery, consider the following two points to prevent central nervous system infection: (1) prophylactic use of antibiotics should be started within 24 hours of birth, and (2) an immunological factor for control of infection must be considered. Maternally acquired antibodies are typically present for up to 2 months after birth. If the infant is breastfed, immunoglobulin M antibodies will remain during the course of breast-feeding. If the repair of the MMC is delayed beyond 2 months postpartum, then serum gamma globulin levels should be obtained, and if the level is decreased, intravenous immunoglobulin can be given prior to surgery (Nielsen-Canarella S, personal communication, 1998). The authors, for surgical purposes, divide MMCs into three types based on anatomical differences. In the first type, the spinal cord, often buckled, travels inside the meningocele sac, and its neural placode surfaces along the dome of the sac. The placode is covered by a thin layer of poorly developed epithelial tissue but not by meningeal lining. The farther caudal part of the spinal cord often enters the spinal canal with defective laminae. The caudal end of the spinal cord or filum is either loosely or densely adhesive to the surrounding tissue. It is important to release the spinal cord from adhesion because a later occurrence of TCS is expected when the bucked spinal cord straightens as the vertebral column rapidly grows. The second type of MMC is characterized by a spoon-shaped neural placode exposed to the surface without meningeal lining. The spinal cord terminates at the caudal extremity of the placode. Postoperative cord tethering may occur from adhesion of the spinal cord to the surrounding meninges or extradural fibrous tissue if meningeal closure is not complete. In the third type, the spinal cord terminates abruptly inside the meningocele sac and the central canal is open to the CSF space. The nerve roots exiting near the caudal extremity surround the cord and are distributed to the meningocele sac. An incomplete form of the terminal ventricle may be present. Meningeal closure of the caudal end is essential for preventing development of TCS. Type 1 Myelomeningocele Under general endotracheal anesthesia intubation, the patient is placed prone and padded with a roll on each side of the trunk. An incision is made immediately outside the junction of the skin and membranous sac in a circumferential manner (Fig. 11.1 A,B). Careful microscopic dissection of the sac from the overlying skin allows for isolation of the neural plaque along its edges. The neural plaque is an embryological dorsal cleft and has no meningeal lining in this type of MMC. The very thin, disorganized epithelial membrane covering the plaque is washed with saline irrigation and trimmed with microscissors. This prevents the enclosure of contaminated devitalized squamous elements under the skin coverage for the repair. Prior to dissection of the sac, it is often necessary to incise the dura longitudinally 0.5 to 1.0 cm cephalad or caudad to its base (Fig. 11.1C). The arachnoid membrane can now be easily identified where its dorsal aspect ends, cephalic and occasionally caudal to the neural plaque. The lateral extension of the arachnoid membrane can be seen everted on each side beyond its fusion with the pia, dorsolateral to the exit of the posterior nerve roots. When the skin covers the major portion of the meningocele sac, the arachnoid extends to underlie the subcutaneous tissue (Fig. 11.1D,E). As soon as the meningocele sac is ruptured, CSF is drained out of the sac causing it to collapse immediately. The spinal cord sinks in the collapsed subarachnoid space and relaxes (Fig. 11.1C). The superficial edge of the meningocele sac is carefully dissected from the lateral end of the neural placode. The arachnoid membrane, which can be found everted lateral to the spinal canal, is then freed from the underlying dura. The dura that is everted over the lateral mass of the vertebrae is also isolated from the facet and fascia. Fibrous tissue around the caudal end of the spinal cord is then dissected free. Next, several 8–0 nylon sutures are passed through both pial edges and tied (Fig. 11.1C). This procedure allows formation of a nearly normal spinal cord and central canal contour (Fig. 11.1C). Both edges of the arachnoid flaps are then approximated over the reformed cord using 8–0 nylon sutures (Fig. 11.1C). Repair of the arachnoid membrane in this fashion establishes postoperative CSF circulation in the subarachnoid space and prevents adhesions from forming between the underlying neural tissue and dura or extrameningeal structures. Dural flaps are then freed from the underlying fascia bilaterally and closed with interrupted 6–0 nylon sutures. In some cases where the arachnoid membrane is not well formed around the meningocele sac, the dural flaps may be used directly to cover the spinal cord. At times, it may be difficult to form an adequate dural flap if the lateral mass of the vertebra is wide and protrudes posteriorly. If dural closure is defective (usually at the caudal end), then bilateral fascial flaps are developed to cover the entire meningocele sac for a watertight closure. However, if the arachnoid and dural layers are closed completely, fascial flap closure is not absolutely necessary. It is recommended that at least two of the three layers (the arachnoid, dura, and fascia) should be closed. Finally, the subcutaneous tissue and skin are closed in the standard fashion, with careful attention not to compress the underlying neural elements. If blanching occurs along the edges, further dissection is necessary. Relaxing incisions or Z-shaped skin flaps may have to be utilized, although these techniques are rarely required. Slightly bluish discoloration after closure is tolerated, and sloughing skin or dehiscence rarely ensues. Kyphectomy has been advocated by Reigel7 to prevent skin ischemia caused by the protruding lateral mass of the bifid vertebrae. Fig. 11.1 (A) Photograph of a type 1 myelomeningocele (MMC) as viewed from the outside. The neural placode is exposed dorsally, with no meningeal covering (solid arrows). The midline groove is well identified, and a poorly developed translucent epithelial membrane covers the placode. The central canal (open arrow) is covered by a thin membrane. (B) An elliptical incision surrounds the myelomeningocele. The buckled spinal cord reaches the dome of the MMC where the placode is exposed. The spinal cord ends in the sacral canal with either loose or dense adhesion to the surrounding connective tissue. It is important to release the tethering of the cord. Fig. 11.1 (C) After laminectomy at one level above the spina bifida, a midline dural incision is made to expose the cephalic end of the neural placode. The midline groove of the neural placode is continuous to the central canal. The trimmed edge of the meningocele sac is shown underneath the arachnoid membrane at the cephalic end of the sac, which is assumed to be a continuation of the intermediate arachnoid in the normal spinal canal. The lateral edges of the sac are excluded in the drawing. The arachnoid and the dura are everted away from the placode. The pia mater has been closed with 8–0 nylon sutures. The second figure shows sutures being placed through the arachnoid (dura is indicated by large arrows and the arachnoid by small arrows in. (D) The central placode has sunk to the anterior spinal canal after cerebrospinal fluid (CSF) draining, and the trimmed edge of the meningocele sac borders the placode (white arrows). The everted arachnoid has a broad base and its lateral extension has been isolated from subcuticular tissue (black arrows). The opening of the central canal (open arrow) and the midline groove of the neural placode are clearly seen. CSF draining from the central canal percolates through the thin membrane over the placode, causing constant wetting of the placode surface. The nerve roots directing cephalad (to the right) and exiting through the arachnoid and dura are identified. Fig. 11.1 (E) Transection of the MMC shows the arachnoid membrane extending laterally to undermine the skin. The meningocele sac is fused with the arachnoid except for the area of the membrane directly attached to the placode edge (arrows). SAS, subarachnoid space. Type 2 Myelomeningocele The repair of this type of MMC is similar to that of type 1. In this case, the skin is isolated from the meningocele sac, which is then incised around its junction to the neural placode. The spoon-shaped placode usually has no filum (Fig. 11.2A). The pia is closed with 9–0 nylon sutures, and formation of the spinal cord is completed (Fig. 11.2B). The arachnoid and the dura are closed in separate layers over the newly formed cord. Closure of the meninges is of utmost importance in preventing postoperative cord adhesion Type 3 Myelomeningocele The spinal cord in this type of MMC characteristically has an open central canal at its caudal end that forms the terminal ventricle (Fig. 11.3A). The caudal end of the spinal cord and the nerve roots are carefully dissected from the surrounding membrane and fibrous tissue for untethering (Fig. 11.3B). Next, the pia mater is closed around the end of the spinal cord to complete the closure of the central canal (Fig. 11.3C). The surrounding nerve roots are carefully dissected. The caudal end of the spinal cord is then closed with pial, arachnoid, and dural sutures. The arachnoid, dural, and fascial flaps are closed in a similar fashion as for type 1 MMC (Fig. 11.3D,E). Postoperatively, the infant should be kept in the prone position with a protective covering over the repair site. Feedings may be started within 8 to 12 hours if bowel sounds are audible and the baby can be held by a nurse or parent for feedings.7 Complications subsequent to the initial MMC repair often necessitate a secondary surgery. The morbidities include the following: (1) dehiscence or CSF leakage11; (2) hydrocephalus6,7,11; (3) infection6,7; (4) new TCS or recurrence of TCS1,6,12–14; (5) local adhesive spinal cord dysfunction; and (6) hindbrain dysfunction15 (which is outside the scope of this chapter). Dehiscence, CSF leakage, and ulceration are almost inseparable problems. Further, incomplete meningeal closure healing results in scar formation around the spinal cord and nerve roots. Hydrocephalus, which is frequently associated with MMCs (80%),6 must be treated promptly by ventricular fluid shunting for protection of cerebral function as well as prevention of CSF leakage through the MMC or repaired wound. The incidence of shunt infection after MMC repair is twice as high as for hydrocephalus from other causes.16 Prophylactic antibiotic administration is useful for the prevention of shunt infection.16–18 Shunt infection (with a 2 to 5% incidence rate16,17) almost always results in ventriculitis and can cause secondary infection of the repaired MMC. Fig. 11.2 (A) Photograph of a type 2 MMC with a placode covered by a thin epithelium (black arrows). (B) This type of myelomeningocele has a spoon-shaped placode. The caudal end has no filum terminale. The pial sutures reform the caudal spinal cord, and the arachnoid and dural sutures complete the covering of the reformed cord The infection rates of MMC repair are variable (12% overall).19 There are several reasons for postrepair infection. MMCs are contaminated soon after the patient’s birth, and organisms can invade the underlying structures through a thin membranous portion. Therefore, the infection is most likely related to the growth of organisms left in the membrane. When CSF leakage persists, organisms spread easily inside the subarachnoid space, resulting in meningitis. Meningitis can also occur secondary to shunt infection or septicemia from other serious infections in the newborn. Fig. 11.3 (A) Drawings of a type 3 myelomeningocele (MMC). (B) Transection of the most cephalic part of the spinal cord shows a central canal. (C) The caudal end of the spinal cord is located in the MMC. The nerve roots arise from the spinal cord outside the central canal in the cerebrospinal fluid (CSF) space and terminate in the sac. (D) Pial sutures around the central canal close the spinal cord. Sutures through the arachnoid edges followed by (E) sutures through the dural and fascial flaps complete the watertight closure. The infection rate has some bearing on the timing of MMC repair. Data reported by McLone and Naidich6 show a significant difference in infection rates between early and late repair: 7% after repair within 48 hours of birth compared with 37% after repair later than 48 hours. The authors advised repair of the MMC within 48 hours, followed by prophylactic antibiotic treatment. These data suggest that repeated contamination of the MMC contributes to postoperative infection, which also results in scar formation adjacent to neural elements. The patient is more likely to develop postoperative cord adhesion if10 (1) the spinal cord or nerve roots were not dissected completely from the surrounding scar and meningeal tissue, (2) dissection resulted in local ischemia, (3) the nerve structures are not covered by the meningeal structure or its inert substitute, or (4) dermal tissue is left under the skin closure.20 Therefore, meticulous repair of neural tissue and meningeal structures cannot be overemphasized to minimize postoperative neurological complications. Due to their similarities in embryonic development, signs and symptoms, and surgical indications and procedures, both lumbosacral lipomas (leptomyelolipomas) and LMMCs are discussed together. Quite often these anomalies are considered to be the mechanical cause of cord tethering, and surgical untethering results in the alleviation of symptoms.15,21–23 The authors’ purpose in this part is to distinguish stretch-induced lesions associated with lipomas and LMMC from those due to spinal cord or nerve root compression and neuronal dysgenesis. Therefore, the discussion is limited to only those patients who underwent redox studies of cytochrome a,a3 and who had proven impairment of oxidative metabolism in the cord segments proximal to the lipoma or LMMC that improved after untethering procedures, thus indicating that these patients had TCS. The definition of LMMCs24 is often unclear because of the complexity of the anatomical arrangement of the spinal cord, nerve roots, and meninges. This is true particularly in the variation of the interface between the spinal cord and lipomatous (fibrolipomatous or fibroadipose) tissue and its relationship to meninges and subarachnoid space. Mainly, this complexity is derived from the disarray of the perineural tissue (mesodermal and ectodermal) in relation to the neurulation abnormality,25,26 the timing of the caudal mass formation, and the arachnoid and dural development.3,27 Chapman21 classified lipomas (leptomyelolipomas) into three types: caudal, dorsal, and transitional. In addition, he provided discrete diagrams to show that lipomas protrude outside the confinement of the arachnoid and dural canal (Fig. 11.4A,B,D). Others28,29 added a filar type, which has been considered synonymous with the tethered spinal cord. French30 defined lipomas as a fibroadipose mass located in the subarachnoid or intradural space (Fig. 11.4C), as opposed to LMMC in which a lipomatous mass protrudes external to the arachnoid and dura. Schut et al22 have found that almost all LMMCs are fluctuant, indicating that CSF is contained in the mass. The authors have modified Chapman’s lipoma model21 and classify LMMCs into three types, all of which have subarachnoid space extension into the lipomatous mass.
Tethered Cord Syndrome Associated with Myelomeningoceles
Anatomical Considerations
Treatment
Objectives of Repair
Preoperative Considerations
Surgical Anatomy
Surgical Technique
Postoperative Management and Complications
Tethered Cord Syndrome Associated with Lipomas and Lipomyelomeningoceles
Classifications of Lipomas and Lipomyelomeningoceles
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







