The Anterior Cerebral Artery
Key Points
Selecting the A1 segment with a wire and microcatheter is often very difficult. Therefore, to force the microcatheter to make this difficult turn, a great deal of forward tension will build up in the microcatheter. Be attentive to the tip of the wire lest a small medial lenticulostriate or cortical branch might perforate as the microcatheter finally advances.
Do not underestimate the capacity of the A1–A2 junction to occlude itself during an endovascular procedure. Anticipate this scenario and think of back-up strategies ahead of time, for example, bilateral carotid catheters, balloon reconstruction assistance, balloon angioplasty.
The anterior cerebral artery arises from the medial aspect of the internal carotid bifurcation below the anterior perforated substance. It courses anteromedially toward the interhemispheric fissure. The usual route that it takes is above the optic chiasm or nerve, meaning that the optic structures therefore slope through the ring of the circle of Willis. At the level of the interhemispheric fissure, the anterior cerebral artery is joined to its contralateral counterpart via the anterior communicating artery in the suprachiasmatic cistern. The anterior communicating artery represents the most common site of intracranial aneurysms, accounting for approximately 30% in most series (1).
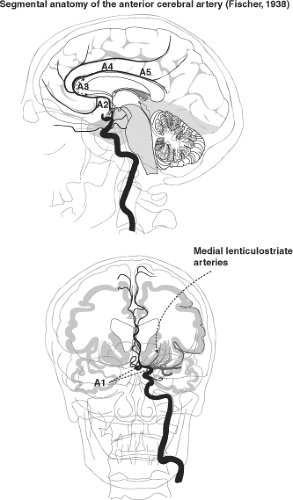
Segmental Anatomy of the Anterior Cerebral Artery
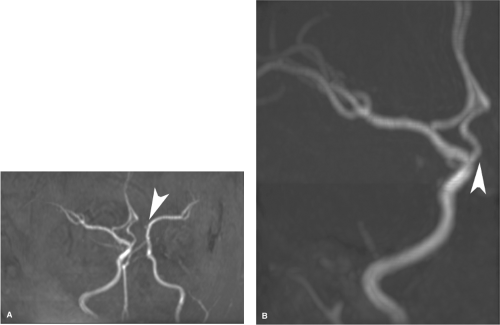
The A1 segment of the anterior cerebral artery extends from the internal carotid artery bifurcation to the anterior communicating artery; the A2 segment extends from there to the junction of the rostrum and genu of the corpus callosum; the A3 extends around the genu until the artery turns sharply posteriorly. The A4 and A5 segments above the corpus callosum are separated by the plane of the coronal fissure (2,3,4) (Figs. 11-1 and 11-2).
Variations of the Anterior Cerebral Artery–Communicating Artery Complex
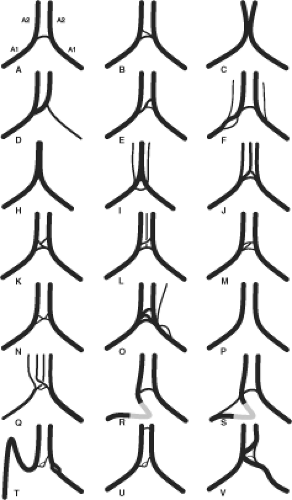
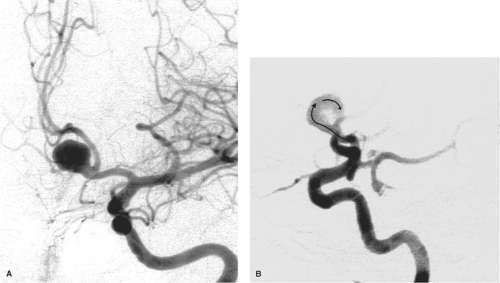
Variations in the region of the anterior communicating artery are not just common, but are to be expected. The term anterior cerebral artery–communicating artery complex is frequently used in clinical parlance. As elsewhere in the circle of Willis, alterations in hemodynamics due to the presence of anomalous vessels are thought to explain the high-frequency vulnerability in patients with aneurysms in this region. The extreme variability of the anatomy in this region, due to redundancy of vessel loops, vessel duplication, and early bifurcation of the A2 segment, can make the anterior communicating artery complex one of the most difficult areas to evaluate angiographically (3) (Figs. 11-3–11-6). Fortunately, 3D angiography has eliminated the need for performing multiple DSA runs in varying obliquity in the hope of finding an optimal profile view of an anterior communicating artery or aneurysm.
Usually, the anterior cerebral arteries join the anterior communicating artery superior to the optic chiasm (70%). When the A1 segments are longer or redundant, this union may occur more anteriorly above the optic nerves. Fenestrations or duplications of the A1 portion of the anterior cerebral artery are common. More rare anomalies include an interoptic or infraoptic course of the A1 segment (5,6,7,8,9,10), or even perforation of the optic nerve by the A1 segment (11). One possible embryologic explanation for an infraoptic course of the anterior cerebral artery suggests that the A1 segment in these instances represents a persistence of the ventral primitive ophthalmic artery taking origin from the internal carotid artery and flowing retrogradely to the anterior cerebral artery.
Variations in the relative size of the A1 segments and the anterior communicating artery are common. Using a diameter of 1.5 mm or smaller as a definition for hypoplasia, approximately 10% of brains demonstrate hypoplastic A1 segments (3,12,13). The anterior communicating artery varies in length up to 7 mm, may be quite tortuous, and measures up to 3.4 mm in diameter. There is a positive correlation between the diameter of the anterior communicating artery and the degree of asymmetry of the A1 segments. This is to compensate hemodynamically for hypoplasia of the smaller vessel. Asymmetry of the A1 segments has an important impact on the likelihood of aneurysm formation in the anterior communicating artery region (Fig. 11-7). The end-on hemodynamic impact of pulsatile flow in the larger A1 segment directed against the anterior wall of the anterior communicating artery is thought to explain the higher rate of aneurysm formation under these circumstances. Up to 80% of anterior communicating artery aneurysms have significant A1
asymmetry. This hemodynamic force also explains why approximately 70% of such aneurysms here project anteriorly (14,15) (Figs. 11-8–11-10). Altered hemodynamics in the anterior communicating artery after therapeutic occlusion of an internal carotid artery are also associated with an increased likelihood of delayed aneurysm formation.
asymmetry. This hemodynamic force also explains why approximately 70% of such aneurysms here project anteriorly (14,15) (Figs. 11-8–11-10). Altered hemodynamics in the anterior communicating artery after therapeutic occlusion of an internal carotid artery are also associated with an increased likelihood of delayed aneurysm formation.
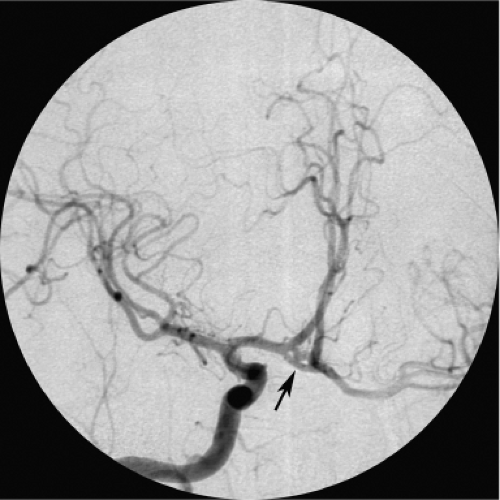
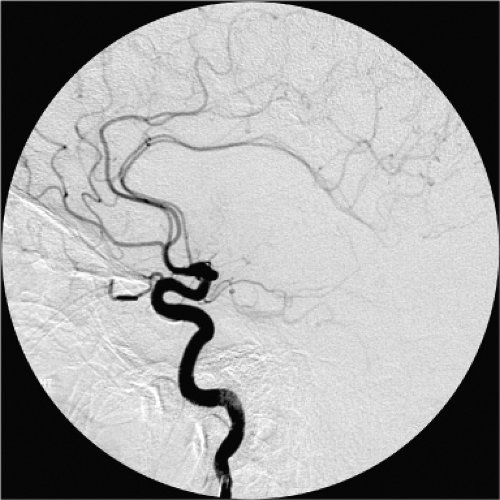 Figure 11-2. Isolated anatomy of the anterior cerebral artery. A lateral view from an internal carotid artery injection in the setting of acute ischemic stroke treatment for complete occlusion of the middle cerebral artery affords an uninterrupted view of the territory and branches of the anterior cerebral arteries in the midline, similar to that depicted in Figure 11-1. |
Other common variations in this area include the presence of two or more anterior communicating arteries in 11% to 43% of brains (14,16,17). This may take the form of a fenestration of the anterior communicating artery or complete duplication or triplication (3,18). Another angiographic difficulty in imaging this area occurs when the anterior cerebral arteries are not exactly parallel; the anterior communicating artery must run an oblique course to connect the two. This calls for flexibility in one’s positioning for imaging of this region.
A rare variant of the proximal anterior cerebral artery is a persistent olfactory artery that runs anteriorly from the internal carotid artery bifurcation along the olfactory groove. It may supply the territory of the anterior cerebral artery distally (19). Fenestrations of the distal A1 segment, an anomaly that is embryologically difficult to explain in this location, may represent an incomplete fusion of the anterior cerebral artery with the primitive olfactory artery (20).
Branches of the Proximal Anterior Cerebral Artery
Anterior Perforator Vessels
From the A1, proximal A2, and anterior communicating artery, two groups of perforator branches are seen. An inferiorly directed group supplies the optic chiasm and nerve. A superiorly directed group of medial lenticulostriate arteries supplies the anterior hypothalamus, septum pellucidum, the medial part of the anterior commissure, the pillars of the fornix, and the anterior aspect of the striatum. Between 2 and 15 basal arteries arise from the A1 segment, usually from the superior and posterior surfaces, particularly from the lateral half of the A1 segment (3). They enter the anterior perforated substance in the basal forebrain (21). Aneurysms of the A1 segment of the anterior cerebral artery, proximal to the anterior communicating artery, have a high likelihood of being related either to a lenticulostriate origin or cortical branch from this segment of vessel or to a fenestration of the vessel (22).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree