Disorder of the CSF dynamics
Cause
Neuroendoscopic treatment
Yes/no
Neuroendoscopic techniques
Neuroendoscopic foraminoplasty of foramen of Monro (NEFPFMO)
Neuroendoscopic septostomy (NESS)
Neuroendoscopic aqueductoplasty (NEAP)
Neuroendoscopic third ventriculostomy (NETV)
Neuroendoscopic lamina terminalis fenestration (NELTF)
Neuroendoscopic foraminoplasty of foramen of Magendie (NEFPFMA)
Neuroendoscopic foraminoplasty of foramen of Luschka (NEFPFL)
Neuroendoscopic fourth ventriculostomy (NEFV)
Disorder of the CSF production
Hyperproduction
Choroid plexus papilloma
No
Hypoproduction
No
Disorder of the CSF flow
Obstruction of foramen of Monro
Congenital atresia or stenosis; ventriculitis; tumor; subarachnoid hemorrhage; choroid plexus hyperplasia or cyst; AVM of the choroid plexus; basilar artery aneurysms or ectasia; functional changes following ventriculoperitoneal, ventriculoatrial, or lumbo-peritoneal CSF shunts; post-NETV; Arnold-Chiari malformation
Yes
(+)
(+)
0
0
0
0
0
0
Obstruction of aqueductus sylvii
Yes
0
0
(+)
(+)
(+)
0
0
0
Obstruction of fourth ventricle foramina
Yes
0
0
0
(+)
(+)
(+)
(+)
(+)
Obstruction of the transition between spinal and cranial subarachnoid space
Yes
(+)
(+)
Disorder of the CSF absorption
Obstruction of the arachnoid villi
Infection or hemorrhage
No
Venous hypertension
Sinus thrombosis or pseudotumour cerebri
No
2.3.1 Neuroendoscopic Treatment of the Disorders of CSF Production
Currently neuroendoscopic treatment of the disorders of CSF production does not exist.
2.3.2 Neuroendoscopic Treatment of Disorders of CSF Flow
The historical trends of neuroendoscopic surgical technique in the treatment of hydrocephalus were thoroughly discussed by the authors in a previous paper [17].
2.3.2.1 Neuroendoscopic Treatment of Obstruction of Foramen of Monro: Unilateral or Bilateral
Neuroendoscopic Foraminoplasty of Foramen of Monro (NEFPFMO)
Indications. NEFPFMO, although infrequently applied in clinical practice, represents the most physiologic surgical approach in the treatment of isolated obstruction or stenosis of foramen of Monro caused by membranous occlusion of the interventricular foramen, leading to restoration of the natural CSF flow. In cases with unclear ventricular landmarks and difficult intraoperative orientation, neuronavigation is extremely valuable in defining the margins of obstructed foramen of Monro and the neighboring eloquent neurovascular structures, thus preventing procedure-related injuries.
Operative technique. The surgical technique of NEFPFMO is straightforward without any significant nuances. The burr hole for NEFPFMO is usually positioned 2–3 cm laterally to the midline and 2–3 cm in front of the coronal suture. After penetration of the dilated lateral ventricle, the obstructed foramen of Monro could be found by tracing up the choroid plexus from the body of the lateral ventricle to its passage to the third ventricle, just behind the fornix. Another landmark is the junction point of the choroid plexus, the thalamostriate vein and the septal vein. However, in complicated cases neuronavigation could be a very useful adjunct. The fenestration of the membrane is usually bluntly performed with or without prior coagulation of the ependyma. The next step is the dilatation with a micro balloon catheter. In patients with high risk of re-occlusion, the implantation of interventricular stent is reasonable and must be judged.
Clinical experience. NEFPFMO is not frequently reported in the literature.
In 1996, Mohanty et al. [42] reported a case of unilateral hydrocephalus caused by membranous occlusion of the ipsilateral foramen of Monro. NEFPFMO was successfully performed simultaneously with fenestration of septum pellucidum.
In 1999, S. Oi et al. [58] in their series described one case with post-shunt monoventricular unilateral hydrocephalus handled effectively by NEFPFMO and neuroendoscopic septostomy (NESS). S. Oi, first in the literature, introduced the term “foraminal plasty of foramen of Monro.”
In 2000, T.T. Wong and and L.S. Lee [85] reported two cases of membranous occlusion of the foramen of Monro with a consequent lateral ventricle dilatation as a complication of ventriculoperitoneal CSF drainage. The patients underwent NEFPFMO of the affected foramen of Monro (in one case NEFPFMO of both foramina of Monro) and neuroendoscopic third ventriculostomy (NETV). The result was excellent in one case, and shunt insertion followed in the other.
In 2001, J. Javier-Fernandez et al. [33] described an adult patient with isolated monoventricular unilateral hydrocephalus due to the obstruction of the foramen of Monro by a thin membrane. NEFPFMO was performed successfully.
In 2007, H. Moriet al. [43] described a case of endoscopic stent placement for treatment of secondary bilateral occlusion of the Monro foramina following endoscopic third ventriculostomy in a patient with aqueductal stenosis. From the very beginning of her clinical history, the patient was treated by ventriculoperitoneal shunt for tri-ventricular hydrocephalus due to aqueductal stenosis. Six years later an MR imaging demonstrated slit-like ventricle with the ventricular catheter and dilated contralateral and third ventricles. NESS and NETV were performed with excellent postoperative clinical recovery and with restoration of the normal ventricle configuration on control postoperative MR images. However, 2 years later the patient’s complaints reappeared and MRI revealed dilatation of both lateral ventricles with a patient stoma on the third ventricle floor. The patient underwent re-NESS and NEFPFMO by fiberscope as well as implantation of stent through one of the foramina of Monro with excellent clinical and radiological recovery at 3-year follow-up.
In 2008, S. Oi and and Y. Enchev [52] in their detailed study of NEFPFMO in the treatment of isolated unilateral hydrocephalus presented two personal cases, thoroughly reviewed the literature, and analyzed the applied surgical techniques. S. Oi and Y. Enchev described the first neuronavigational NEFPFMO performed alone without any additional neuroendoscopic procedure. The neuronavigation was used to point the most appropriate entry point and to outline the borders of the obstructed foramen of Monro (Fig. 2.1).
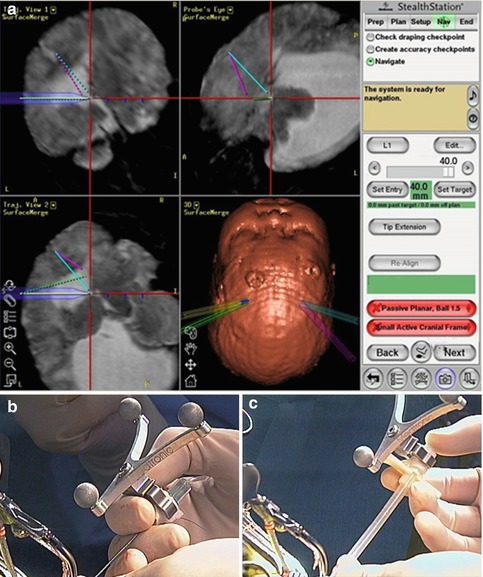
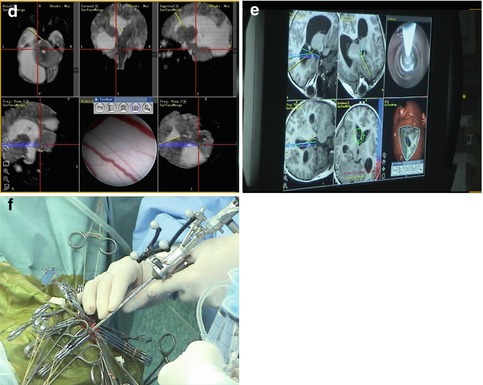


Fig. 2.1
Navigational neuroendoscopic foraminal plasty of foramen of Monro, clinical case. (a, d, e) Navigational guidance to the occluded foramen of Monro. (b, c) The ventricular puncture needle and the transparent peel-away sheath with the navigational reference star. (f) Freehand maneuver of a small-diameter rigid-rod neuroendoscope [56] with the navigational reference (Reproduced with permission from Enchev and Oi [17])
In 2008, De Bonis et al. [13] presented an adult patient with idiopathic occlusion of the foramina of Monro. NEFPFMO was performed unilaterally due to the observed wide fenestration of septum pellucidum. At the end of the procedure, an intraventricular catheter with subcutaneous Rickham reservoir was inserted. Six months later the patient was without any complaints and follow-up MRI demonstrated gradually decreasing ventricular size.
In 2010, Sharifi et al. [74] described a case of adult post-shunt bilateral occlusion of foramina of Monro treated successfully by simultaneous NESS and bilateral NEFPFMO.
In 2011, S. Kalhorn et al. [36] reported a case of an idiopathic bilateral stenosis of the foramen of Monro treated effectively by image-guided NEFPFMO and NESS. The authors approached the narrower lateral ventricle in order to enable a safer penetration into the contralateral frontal horn during NESS.
In 2012, El Refaee et al. [14] published a unique case of bilateral occlusion of the foramina of Monro as a consequence of third ventriculostomy performed with clinical success 30 months ago. Since the septum pellucidum was perforated as a result of chronic hydrocephalus, the patient underwent unilateral NEFPFMO together with implantation of a silicon stent through the restored interventricular foramen. To prevent the intraventricular stent migration, it was fixed to subcutaneous Rickham reservoir. Ten months postoperatively, the clinical and image result was excellent.
Conclusion. In the cases with unilateral or bilateral obstruction or stenosis of foramen of Monro, NEFPFMO as a stand-alone procedure or in combination with NESS represents safe and reliable treatment option.
Neuroendoscopic Septostomy (NESS)
Indications. NESS is indicated in patients with isolated monoventricular hydrocephalus due to unilateral obstruction of the foramen of Monro associated with distorted intraventricular anatomy landmarks of the effaced foramen and thus expected risky NEFPFMO. If both foramina of Monro are occluded and septum pellucidum is intact, NESS is preferable to bilateral foraminoplasty because the former procedure is technically easier and the latter has the risk of bilateral fornix injury. In symptomatic cavum septum pellucidum, NESS is the treatment of choice.
Operative technique. NESS could be performed as a single procedure or simultaneously with NEFPFMO. In cases indicated for NESS alone, the burr hole is usually positioned in front of the coronal suture and 5–6 cm off the midline, ipsilaterally to the enlarged ventricle. Frameless stereotaxy could be useful in defining the optimal position of the entry point, in guiding the trocar through the parenchyma and in selecting the most secured fenestration site. Usually, the septostomy is performed bluntly in a thin avascular part followed by balloon dilatation. The eventual bleeding from septum pellucidum vessels could be controlled by continuous irrigation or by bipolar cautery. The resulted communication between both lateral ventricles must be wide enough to reduce the risk for re-occlusion.
Clinical experience. NESS is more frequently reported in the literature than NEFPFMO.
In 1991, C. Heilman and A. Cohen [30] described two NESS performed by the so-called saline torch. In the follow-up period, no additional treatment was required.
In 1994, M. Walker et al. [82] reported a series of nine NESS. Unsatisfactory results, in long follow-up period, were attained in only two patients.
In 1998, M. R. Gaab and H. W. Schroeder [22] described 4 patients with NESS. In 10 months mean follow-up reduced ventricular volumes were observed in all cases.
In 1999, three series of NESS were published. In the study of M. Gangemi et al. [24], five patients with monoventricular hydrocephalus underwent NESS. Within a follow-up period of almost 3 years, the clinical results were excellent in all cases, which were asymptomatic.
S. Oi et al. [58] reported five cases with different forms of hydrocephalus successfully treated by NESS (with clinical and radiological improvement at follow-up).
J. U. Choi et al. [7] described ten patients with NESS for obstructed ventricles due to various pathologies. The authors found that NESS makes shunting possible and reasonable in all cases.
In 2002, M. Fritsch and M. Mehdorn [20] in their paper “Endoscopic intraventricular surgery for treatment of hydrocephalus and loculated CSF space in children less than 1 year of age” presented a case of failed NESS with a consequent CSF shunt insertion.
In 2003, H. Hamada et al. [29] reported a series of 20 patients with isolated monoventricular hydrocephalus treated by NESS. NESS was unsuccessful in two adult patients due to thick septum pellucidum and limited working space. NESS was repeated in two children. In the remaining patients, good results were achieved without septostomy-related morbidity.
In 2003, P. Aldana et al. [1] published their experience with 43 NESS in 32 patients. During the mean follow-up of 2.5 years, the primary septostomies persisted in more than half of the cases. Ten patients underwent second NESS following the re-occlusion of their initial septal fenestration, with clinical success in 9 of them. The repeat NESS on the last follow-up revealed septostomy failure rate of less than 20 %. Based on these results, it was summarized that NESS represents effective and reliable treatment option for both initial and recurrent isolated lateral ventricle hydrocephalus, preventing insertion of CSF shunts.
In 2004, T. Beems and J. Grotenhuis [3] in their study of long-term complications of neuroendoscopic procedures presented a series of 21 NESS with or without NETV with 95 % success rate. Procedure-related complications were 10 % and mortality rate 0 %.
In 2008, S. Oi and Y. Enchev [52] described a case of congenital isolated unilateral hydrocephalus successfully treated by NESS in combination with NEFPFMO.
In 2009, J. Oertel et al. [45] in their series of 134 neuroendoscopic procedures in children presented 13 NESS in 12 patients. NESS was associated with NETV in five cases, with tumor biopsy in 3, with stent insertion in 2, with cystoventriculostomy in 1, and with aqueductoplasty in one. Clinical and radiological benefit was achieved in 6 patients. Four patients became shunt dependent. Clinical and radiological success rate was worst in children younger than 6 months of age.
In the same year, same authors [46] described a series of 32 NESS performed in 30 patients. The CSF flow disorder was caused by neoplasm (16 cases), multiloculated cystic hydrocephalus (8 cases, including 2 revisions), septum pellucidum cysts (3 cases), membranous or inflammatory isolated lateral ventricles (3 cases), and giant aneurysms (2 cases). NESS was combined in one stage with 13 endoscopic tumor biopsies/resections, 9 NETV, and 9 other neuroendoscopic procedures. During the mean follow-up period of 26 months, 26 patients improved clinically.
In 2011, F. Vaz-Guimaraes Filho et al. [80] reported seven adult patients with unilateral hydrocephalus, selected out of their almost 800 neuroendoscopic procedures in a 15-year period. All but one case (6/7) were with intraventricular cysticercosis and one (1/7) with congenital stenosis of foramen of Monro. NESS restored the CSF flow in all seven patients with uneventful postoperative period and excellent clinical outcome during the mean follow-up of 66 months.
In 2012, G. Tamburrini et al. [77] described a series of 63 patients (50 adults and 13 children) which underwent NESS during the period of 10 years. In 46 of the patients, all adults, hydrocephalus was due to neoplasm; in 11 the etiology was postinfectious and/or posthemorrhagic; and in the remaining 6 patients, hydrocephalus was malformative. The authors succeeded to perform NESS in all but 2 posthemorrhagic cases, presenting with a dense multilayered septum; 37 out of 63 patients underwent simultaneously one or more additional neuroendoscopic procedures. During the mean follow-up of 2 years, all but one patient demonstrated clinical and radiological improvement.
Conclusion. NESS is a reasonable treatment option for selected cases of isolated lateral ventricle with negligible procedure-related morbidity and mortality. Hence, NESS as a stand-alone procedure or in combination with NEFPFMO must be the treatment of choice of these forms of disordered CSF dynamics. Neuronavigation is usually dispensable. Difficulties in performing NESS could be expected in postinfectious ventricle causing thickening of the septum.
2.3.2.2 Neuroendoscopic Treatment of Obstruction of Aqueductus Sylvii
Neuroendoscopic Aqueductoplasty (NEAP)
Indications. NEAP with or without aqueductal stenting is indicated in cases with membranous aqueductal stenosis. NEAP could be performed alone or in combination with NETV.
Operative technique. The position of the entry point is selected based on detailed analysis of CT and especially MR images or with the aid of neuronavigation. Most frequently its coordinates are about 2 cm off the midline and 3–5 cm in front of the coronal suture, depending on the width of the foramen of Monro. Following the inspection of the floor of the third ventricle and the proximal part of the aqueduct, the NEAP is performed by the tip of Fogarty balloon catheter. If it is necessary a delicate dilatation of the perforation could be accomplished by inflation of the micro balloon. Further inspection of the distal aqueductal part and the foramina of the fourth ventricle could be performed by flexible neuroendoscope but with substantial risk for iatrogenic injury of the tectal region.
Clinical experience. NEAP is one of the infrequent neuroendoscopic procedures in treatment of disordered CSF flow. The idea for aqueductoplasty belonged to Dandy [11] as he was the first to perform aqueductal reconstruction by open surgery in 1920.
In 1993, Oka et al. [60] described a series of seven anterograde NEAP, performed by flexible neuroendoscope, simultaneously with NETV in patients with obstructive hydrocephalus due to aqueductal stenosis. Procedure-related complications were not observed. All but one patient did not require CSF shunt insertion during the follow-up period.
In 1999, Oi et al. [58] reported the first 2 cases with NEAP as a stand-alone procedure for distal membranous aqueductal stenosis. One of the patients underwent anterograde and the other retrograde NEAP. However, subsequent restenosis occurred.
In 1999, H. W. Schroeder and M. R. Gaab [70] presented a heterogeneous series of 17 NEAP: NEAP as a stand-alone procedure, NEAP in combination with NETV, and NEAP plus subsequent aqueductal stenting. In 6 of the patients diplopia was observed as a procedure-related complication. Within the mean follow-up of 1.5 years, the clinical results were satisfactory in all but 6 of the cases.
In 1999, C. Teo et al. [78] described their experience with neuroendoscopic treatment of trapped fourth ventricle, including 4 NEAP, one of them via suboccipital approach. In two of the cases restenosis was successfully treated by combination of NEAP and aqueductal stenting.
In 2004, M. Fritsch et al. [21] reported 8 patients with NEAP as a stand-alone procedure and 5 patients with NEAP plus aqueductal stent implantation. In the first group 6 restenoses were observed. However, in the second group no complications were found.
In 2004, H. Schroeder et al. [72] described a series of 39 NEAP in 33 patients with noncommunicating hydrocephalus due to aqueductal stenosis. NEAP was combined with NETV in 13 patients and with aqueductal stenting in 1. The success rate in the whole group was 76 % and higher (82 %) in the group with NEAP alone. In 22 patients radiological improvement was noticed. During the follow-up period of 40 months, aqueductal restenosis occurred in seven cases. The authors concluded that even the initial success rate of NEAP was comparable to that of NETV; with longer follow-up the aqueductal restenosis rate became higher.
In 2005, J. Sansone and B. Iskandar [65] presented 11 NEAP by trans-foramen magnum trans-fourth ventricle approach in 9 patients with membranous aqueductal stenosis. At the end of the almost 2-year-long follow-up period, all the patients were symptom-free. One case underwent two subsequent re-NEAP procedures as an attempt to treat the restenosis. However, aqueductal stenting was needed ultimately. The authors recommended their technique to be applied by experienced neuroendoscopist for selected cases.
In 2005, Gavish et al. [25] reported a series of 5 NEAP through a “tailored craniocervical approach.” The selected patients presented with a distal membranous aqueductal stenosis. The postoperative course was uneventful with excellent clinical and radiological outcome in all patients. Based on these excellent results, the authors advocated their approach as a reliable alternative to other neuroendoscopic techniques.
In 2006, G. Cinalli et al. [9] described 7 complicated cases with posthemorrhagic and postinfectious loculated hydrocephalus previously treated by CSF shunting and in 5 of them by other neuroendoscopic procedures for supratentorial isolated compartments. Three of the patients underwent NEAP alone and the remaining 4 NEAP plus aqueductal stenting. Supratentorial approach was used in 5 cases and infratentorial in 2. All patients improved clinically and radiologically. Two of the patients without aqueductal stent experienced restenosis.
In 2009, J. Oertel et al. [45] reported 14 NEAP in 14 children. NEAP was combined with NETV (9 cases), aqueductal stenting (5 cases), NESS (1 case), and tumor removal (1 case). Excellent outcome was achieved clinically in 57 % and radiologically in 43 %. Restenosis of the cerebral aqueduct was confirmed in 3 patients. During the follow-up period, 53 % needed CSF shunting. Permanent procedure-related complications were observed in 2 patients.
Conclusion. NEAP is a tricky procedure with significant potential hazards for the fornix, the floor of the third ventricle, and especially for the periaqueductal parenchyma. NEAP is indicated in cases with short or membranous proximal (anterograde NEAP) or distal (retrograde NEAP) aqueductal stenosis as well as in cases in whom NETV is contraindicated due to a dense and thick third ventricle floor or to an atypical position of the basilar tip at the prospective fenestration site. In most of the cases, if available, neuronavigation could be useful for planning and guiding. Aqueductal stenting as an addition of NEAP most probably reduces the restenosis rate.
Neuroendoscopic Third Ventriculostomy (NETV)
Indications. NETV is indicated in any case of obstruction of the CSF flow distal to the floor of the third ventricle, including the patients with aqueductal stenosis and occlusion.
Operative technique. The preoperative analysis of sagittal and coronal MR images is of utmost importance for the precise planning of the burr hole position in a trajectory line with the foramen of Monro and the optimal position of the ventriculostomy. Important consideration is full awareness of the relation between the third ventricle floor and the basilar artery. Neuronavigation is highly recommended if it is available. The entry point is usually 2–3 cm off the midline and in front of the coronal suture. After entering the lateral ventricle, the endoscope is advanced through the foramen of Monro. The floor of the third ventricle is inspected, and the infundibular recess and mamillary bodies are identified (Fig. 2.2). According to the individual anatomical peculiarities, the best position of the stoma is defined in order to prevent neurovascular injury. Usually, the safest perforation site is in the center of the triangle with angles of the mamillary bodies and infundibular recess, just behind the clivus [73]. The blunt perforation by rigid instrument is the preferred method. Schroeder et al. [73] recommend avoidance from perforation of the floor by the tip of balloon catheter, because of the risk of slippage of the tip and eventual consequent neurovascular damage (Fig. 2.3). In cases with dense thick floor in which the blunt perforation could be associated with significant hypothalamic traction, the reasonable approach is the bipolar cauterization. The initial perforation is dilated by Fogarty balloon catheter, inflated with fluid, to dimensions of 3–6 mm in diameter [73]. The underlying interpeduncular and pontine cisterns could be inspected and the Liliequist membrane is perforated.
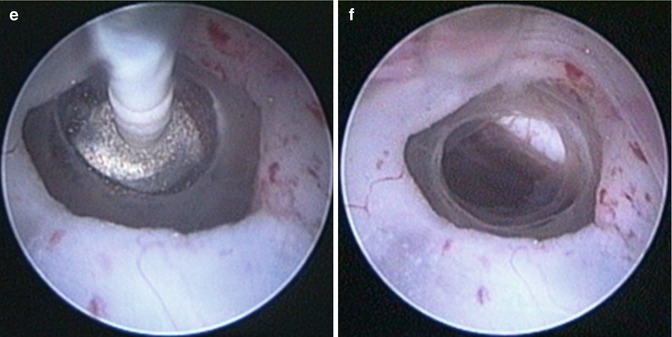

Fig. 2.2
High-resolution neuroendoscopic surgical field approach to the floor of the third ventricle. (a) Note the choroid plexus, septal vein, and the foramen of Monro. (b) Note the landmarks of the third ventricle floor. (c) The floor of the third ventricle with the mamillary bodies and the infundibular recesses. Note the partial transparency of the floor (Reproduced with permission from Enchev and Oi [17])


Fig. 2.3
Neuroendoscopic third ventriculostomy (NETV). Note the fenestration of Liliequist membrane (c–f) as additional and separated procedure after NETV (a, b) (Reproduced with permission from Enchev and Oi [17])
Clinical experience. Currently, NETV is the most frequently performed neuroendoscopic procedure.
The first NETV in history was performed by William Mixter, a urologist, in 1923 [41], using a urethroscope. Despite the technical imperfections, the postoperative course in his child patient with noncommunicating hydrocephalus was uneventful, and the clinical result was excellent.
The next reports of NETV were 12 years later in 1935 and 1936, by J. Scarff [66, 67]. Scarff used a sophisticated endoscope equipped with an electrode for coagulation, system for irrigation, and special tip for performing the third ventriculostomy. Based on his clinical results and autopsy studies, Scarff concluded that simple puncture fenestration was not functionally enough and that further dilatation of perforation was necessary.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








