5
Clinical Evaluation of the Brachial Plexus
Before attempting to examine patients with injuries to the brachial plexus, one should fully understand brachial plexus anatomy. The proper examination techniques for the muscles of the upper extremity and shoulder girdle must also be mastered. In this chapter, I will present this often-daunting anatomical information, discuss lesion localization in the brachial plexus, and provide guidelines for examining patients. The clinical aspects of some common causes of brachial plexus injury will also be described; these should be kept in mind when formulating a differential diagnosis.
 The Proximal Brachial Plexus Palsies
The Proximal Brachial Plexus Palsies
Spinal Nerve Myotomes
Like spinal nerve dermatomes, spinal nerve myotomes are approximations. Despite variations, however, learning spinal nerve myotomes remains important for the clinical evaluation of patients with cervical radiculopathies and proximal brachial plexus lesions. The C5 to T1 spinal nerve myotomes are presented below.
The C5 Spinal Nerve: Arm Raise
The semi-autonomous muscular innervation of the C5 spinal nerve includes arm abduction and external rotation. These two movements are very important for upper extremity function and are classically lost following an upper brachial plexus injury (e.g., birth palsy). The terminal branches mediating these movements include the axillary nerve to the deltoid muscle, and the suprascapular nerve to the supraspinatus and infraspinatus muscles. The supraspinatus (first 30 degrees) and deltoid (30 to 90 degrees) abduct the arm; the infraspinatus is the arm’s most important external rotator (the teres major also externally rotates the arm). A composite movement involving all three of these muscles, and therefore predominantly mediated by the C5 nerve root, is the arm raise. Beginning with the arms straight along the side of the body, the patient abducts them to 90 degrees, while simultaneously externally rotating them so that the undersurface of the upper arm faces forward (Fig. 5-1).
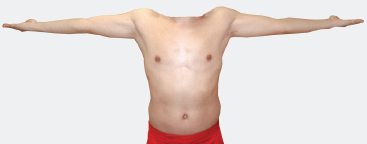
Figure 5-1 Semi-autonomous motor innervation of the C5 spinal nerve: the arm raise. Beginning with the arms straight along the sides of the body, the patient simultaneously abducts and externally rotates the arms to 90 degrees.
The C5 dermatome covers the lateral portion of the shoulder and arm down to the elbow (Fig. 5-2). In part, the upper-lateral cutaneous nerve to the axillary nerve, as well as the lower-lateral brachial cutaneous nerve to the proximal radial nerve carries sensation from this area.
The C6 Spinal Nerve: Chin-up
The semi-autonomous muscular innervation of the C6 spinal nerve includes forearm supination and flexion, as well as arm extension and adduction. The radial nerve caries C6 innervation to the supinator (supination) and brachioradialis (forearm flexion with the forearm partially supinated). The musculocutaneous nerve carries fibers to the biceps brachii (forearm flexion and supination) and brachialis (forearm flexion). The latissimus dorsi extends and adducts the arm via the thoracodorsal nerve, which is primarily C6 mediated (C7 can also provide major innervation to this muscle). A composite C6 body movement would therefore be a classic underhand chin-up. For this movement, the supinated forearm flexes and the latissimus dorsi contracts, pulling one’s chin over the bar (Fig. 5-3). With loss of C6 motor innervation the biceps and brachioradialis reflexes should be absent.
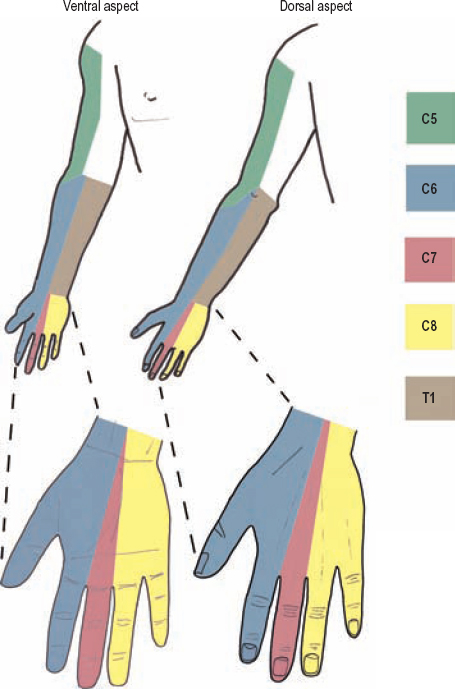
Figure 5-2 Upper extremity (C5-T1) sensory dermatomes. Sensation to the medial upper arm (not shaded) is carried by the T2 spinal nerve via the medial brachial cutaneous nerve.
The lateral forearm and thumb are the sensory territory of the C6 spinal nerve (Fig. 5-2). This sensation is carried in part by the lateral antebrachial cutaneous nerve to the musculocutaneous nerve, and for the thumb, by the terminal sensory branches of both the median and radial nerves.
The Upper Trunk
The upper trunk carries axons from the C5 and C6 spinal nerves. Injury to the upper trunk is commonly caused when the shoulder is forced downward, while the head is simultaneously stabilized or pushed in the opposite direction, thereby stretching the upper portion of the brachial plexus. This mechanism of injury commonly occurs with motorcycle trauma, falls, and birth palsies. An upper trunk injury is referred to as an Erb’s palsy. With this injury, the C5 and C6 innervated muscles are weak. The affected limb assumes a characteristic position at rest secondary to the unopposed action of the remaining musculature. Patients typically have their affected arm adducted and internally rotated (unopposed pull of pectoralis major), forearm extended and pronated (unopposed pull of the triceps and pronator teres), and the wrist and fingers flexed (from weak finger and wrist extensors [variable C6 innervation]). This position is called the waiter’s tip position (Fig. 5-4).

Figure 5-3 Semi-autonomous motor innervation of the C6 spinal nerve: the chin-up. For this movement, the supinated forearm flexes, and the latissimus dorsi contracts; this pulls the chin over the bar.

Figure 5-4 Erb’s palsy or waiter’s tip position waiter’s (simulated). Patients characteristically have the affected arm adducted and internally rotated (unopposed pull of pectoralis major), forearm extended and pronated (unopposed pull of the triceps and pronator teres), and the wrist and fingers flexed (from weak finger and wrist extensors [variable C6 innervation]).
The same mechanisms may cause either an upper trunk or spinal nerve injury. However, weakness involving the rhomboids (dorsal scapular nerve), serratus anterior (long thoracic nerve), and/or diaphragm (phrenic nerve), helps localize the injury to the C5 and C6 spinal nerves, where these branches originate, rather than the upper trunk per se. A lesion involving the upper trunk, or alternatively both the C5 and C6 spinal nerves, causes sensory loss on the lateral half of the arm and forearm, as well as the whole thumb (Fig. 5-5).
The Middle Trunk
The C7 Spinal Nerve: Triceps Push Down
The middle trunk is only made up of fibers from the C7 spinal nerve; hence, these two neural elements will be considered together. The near autonomous muscular control of the C7 nerve root includes the triceps (radial nerve), flexor carpi radialis (median nerve), flexor carpi ulnaris (ulnar nerve), and pronator teres (median nerve). C7 also provides innervation to the wrist extensors, finger extensors, and finger flexors. However, innervation of the latter muscles is either variable, or strongly shared with other nerve roots (e.g., C6, C8); therefore, they are not considered autonomous. The composite movement of the C7 spinal nerve and middle trunk is the triceps pushdown. This movement is made, for example, when you push down on a tabletop when getting up from being seated at a table (Fig. 5-6). For this to occur, one places the forearms in pronation (pronator teres), flexes the wrists (flexor carpi radialis and ulnaris), and contracts the triceps to extend the forearms. A person with a middle trunk palsy cannot do this. It is not possible to differentiate a middle trunk from C7 spinal nerve lesion based on motor examination alone. To do so, one must use other clinical and radiographic findings (e.g., C7 sensory nerve action potential, myelography, spinal nerve lesions present at adjacent levels, etc.).
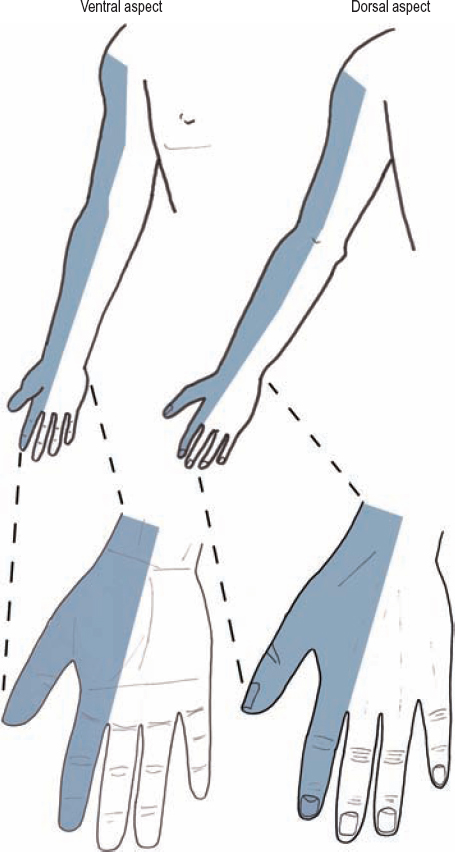
Figure 5-5 Sensory loss for an upper trunk or combination C5/C6 spinal nerve injury. This lesion yields a sensory loss involving the lateral half of the arm and forearm, as well as the whole thumb.
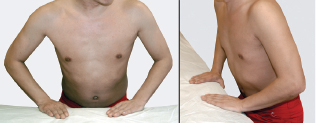
Figure 5-6 Semi-autonomous motor innervation of the C7 spinal nerve: Triceps press down. This movement is made when you push down on a tabletop upon getting up from being seated. For this to occur, one places the forearm in pronation (pronator teres), flexes the wrist (flexor carpi radialis), and contracts the triceps to extend the forearms.
The volar and dorsal aspects of the long finger are almost exclusively within the C7 dermatome (Fig. 5-2). The sensory division of the median nerve (volar aspect of the long finger and its nail bed) and the superficial sensory radial nerve (dorsal aspect of the long finger) carry this sensation. Lesions of the middle trunk, made up solely of C7 fibers, logically cause the same pattern of sensory loss as does a pure C7 palsy.
The C8 Spinal Nerve: Hand Grasp
The C8 spinal nerve provides motor input to many of the long finger flexors (and extensors), as well as to the hand intrinsic muscles, sharing the innervation of this latter group of muscles with T1. Some of the most common muscles to become weak with a C8 palsy include the flexor profundi to the index and long finger (distal interphalangeal joint flexion), thenar intrinsics including the abductor pollicis brevis and opponens pollicis, and extensors to the thumb, forefinger, and long finger. Therefore, an easy way to assess C8 function is to have the patient grasp and let go of your fingers, with special attention paid to the first three digits (Fig. 5-7). A C8 palsy would prevent the patient from performing this movement accurately and strongly.

Figure 5-7 Semi-autonomous motor innervation of the C8 spinal nerve: hand grasp. A quick and easy way to assess C8 function is to have the patient grasp and let go of your fingers, with attention paid especially to the first three digits. A patient with a C8 palsy will have trouble doing this smoothly, strongly, and repetitively.

Figure 5-8 Semi-autonomous motor innervation of the T1 spinal nerve: spreading the fingers. The T1 spinal nerve is best tested in isolation by having the patient spread the fingers. The dorsal interossei muscles control this movement. Atrophy of the first dorsal interosseus muscle, when present, is readily observed.
The C8 dermatome covers the medial, or ulnar, third of the hand, including the fifth digit and lateral hypothenar eminence (Fig. 5-2). The dorsal ulnar cutaneous and palmar ulnar cutaneous nerves, along with the superficial sensory division of the ulnar nerve, carry sensation from this area.
The T1 Spinal Nerve: Spreading the Fingers
The T1 spinal nerve is best tested in near isolation by having the patient spread the fingers (Fig. 5-8). This movement is mediated by the dorsal interossei muscles, which are predominantly T1 innervated. Atrophy of the first dorsal interosseus muscle, when present, is readily observed. The ulnar nerve carries T1 motor fibers down to the dorsal interossei muscles. The T1 sensory dermatome mainly covers the medial half of the forearm (Fig. 5-2), with its sensory fibers being carried by the medial antebrachial cutaneous nerve, a distal branch of the medial cord.
The Lower Trunk
Because the lower trunk is made up of the C8 and T1 nerve roots, injuries of the lower trunk cause marked hand weakness, including problems with hand grasp and finger spreading. Patients with lower trunk injuries classically have good shoulder and elbow function, but report trouble with fine finger movements and grip strength. A lower trunk or combination C8 and T1 spinal nerve lesion causes sensory loss along the medial portion of the forearm and hand, including the fifth digit (Fig. 5-9).
Injury to both the C8 and T1 spinal nerves is called a Klumpke’s palsy. This injury may occur with violent upward pulling of a child’s outstretched arm, or following any type of injury where the upper extremity is suddenly forced upward (e.g., grabbing something when falling, motorcycle accidents). Birth palsy may rarely present as an isolated Klumpke’s paralysis.
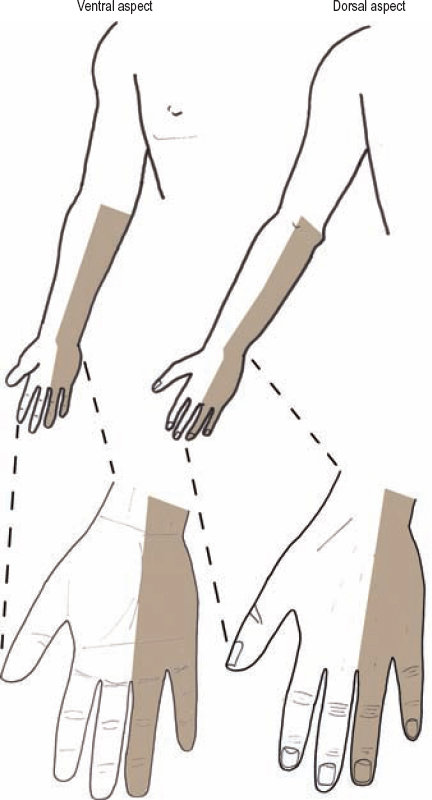
Figure 5-9 Sensory loss for a lower trunk or C8/T1 spinal nerve lesion. This lesion causes a sensory loss in the medial portion of the forearm and hand, including the fifth digit.
Pancoast syndrome is invasion and compression of the lower trunk by an apical lung mass. It usually begins with pain radiating down the inner aspect of the arm and forearm. Approximately one third of patients develop motor and sensory deficits, and two thirds have Horner’s syndrome. Small tumors can be undetectable on X-ray; therefore, a computerized tomography (CT) scan with contrast is often required to confirm the diagnosis.
 The medial arm is predominantly covered by the T2 dermatome. The T3 dermatome includes the axilla and a portion of the proximal, medial arm.
The medial arm is predominantly covered by the T2 dermatome. The T3 dermatome includes the axilla and a portion of the proximal, medial arm.
Summary
Assessment of the proximal brachial plexus (spinal nerves and trunks) should be performed with an understanding of spinal nerve myotomes. Patterns of muscular weakness are used to localize the injury to one or more of the spinal nerves or trunks. Sensory loss of the upper lateral arm (C5), thumb (C6), long finger (C7), fifth finger (C8), and medial forearm (T1) is then used to help confirm your localization. Muscles innervated by branches from the C5 and C6 spinal nerves and upper trunk (long thoracic, phrenic, dorsal scapular, and suprascapular nerves) may be examined and then used to differentiate lesions affecting the upper trunk versus spinal nerves. Supplemental information useful for lesion localization includes myelography to document pseudomeningoceles, and chest radiographs to evaluate diaphragm asymmetry.
 The Distal Brachial Plexus Palsies
The Distal Brachial Plexus Palsies
Plus Palsies
The easiest way to remember the clinical manifestations of a cord lesion is to think of what happens to a patient with a musculocutaneous, ulnar, or radial nerve palsy (the terminal branches of each cord), and then add to these nerve’s respective patterns of weakness any muscles innervated by other branches from the cord in question. I call these plus palsies.
Lateral Cord: Musculocutaneous Plus Palsy
The lateral cord is formed by the anterior divisions of both upper and middle trunks, and therefore, contains fibers from C5, C6, and C7. This cord bifurcates providing the median nerve’s lateral component (C5 to C7), and then continues distally as the musculocutaneous nerve. As expected, an isolated lesion to the lateral cord consists of a musculocutaneous nerve palsy, plus a partial median nerve deficit; that is, a deficit only involving the median nerve’s C5 to C7 portion. This deficit pattern may be termed a musculocutaneous plus palsy (Fig. 5-10).
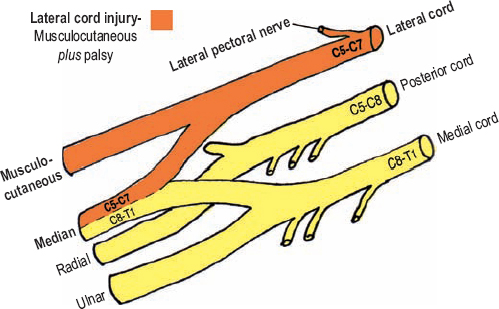
Figure 5-10 Musculocutaneous plus palsy (lateral cord injury). The lateral cord terminates by providing the median nerve’s lateral component (C5-C7), and then continues distally as the musculocutaneous nerve. As expected, an isolated lesion to the lateral cord consists of a musculocutaneous nerve palsy plus a partial median nerve deficit involving its C5 to C7 portion.
The classic musculocutaneous palsy causes forearm flexion weakness secondary to biceps brachii, coracobrachialis, and brachialis weakness, and sensory loss in the lateral forearm (lateral antebrachial cutaneous nerve). As mentioned, median nerve function can be divided into lateral (C5 to C7; lateral cord) and medial (C8, T1; medial cord) components. The lateral cord provides all the median nerve’s sensory fibers (the medial cord does not provide cutaneous sensation to the median nerve). Therefore, lateral palm and first three digit sensory loss occurs with a lateral cord injury. Although the lateral component is primarily sensory, it also controls the more proximal median innervated muscles (pronator teres and flexor carpi radialis). In contrast, the medial (C8, T1) component controls the distal median innervated muscles (hand intrinsics). The long finger flexors (flexor digitorum superficialis and the flexor digitorum profundus to the first two fingers) represent an intermediate gray zone, with contribution from both median nerve components. However, the C8 and T1 contribution is usually greater for these muscles.
Therefore, a lateral cord injury causes a musculocutaneous palsy (forearm flexion weakness), plus forearm pronation and wrist flexion weakness secondary to involvement of the lateral cord’s motor contribution to the median nerve. Sensory loss in the lateral forearm and tips of the first three digits should also be present following a lateral cord injury. Furthermore, weakness of the clavicular head of the pectoralis major should also occur, considering the medial pectoral nerve, which innervates this muscle, originates from the lateral cord.
Medial Cord: Ulnar Plus Palsy
The medial cord is a continuation of the lower trunk’s anterior division, containing C8 and T1 nerve fibers. Although an accessory branch from the lateral cord donating some C7 fibers can occur, this is omitted for simplicity. The medial cord provides the medial component of the median nerve, containing C8 and T1 fibers, and then continues as the ulnar nerve into the arm. Therefore, an isolated medial cord lesion consists of an ulnar nerve palsy, plus the loss of C8/T1 median nerve function (Fig. 5-11).
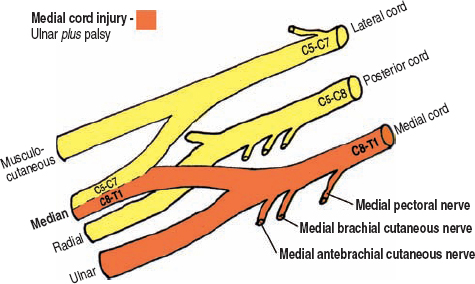
Figure 5-11 Ulnar plus palsy (medial cord injury). The medial cord provides the medial root to the median nerve, containing C8 and T1 fibers, and then continues as the ulnar nerve into the arm. Therefore, an isolated medial cord lesion consists of an ulnar nerve palsy plus weakness in the muscles controlled by the C8 and T1 components of the median nerve.
An ulnar palsy causes weakness in medial wrist flexion (flexor carpi ulnaris), distal interphalangeal joint flexion weakness involving the fourth and fifth digits (flexor digitorum profundus), other fifth digit movements (opponens, flexor, and abductor digiti minimi), and finger abduction and adduction (interossei). Ulnar nerve sensory loss involves the medial third of the hand. As mentioned, the medial component of the median nerve controls the median nerve innervated hand intrinsics, including the opponens pollicis, flexor pollicis brevis (superficial head), abductor pollicis brevis, and the first two lumbricals. Therefore, a medial cord lesion, or ulnar plus palsy, would in addition to causing ulnar motor loss, cause median innervated thumb weakness, as well as difficulty extending the proximal interphalangeal joints of the first two fingers (lumbricals). Of note, these C8/T1 median nerve muscles are the same ones whose innervation is exchanged with a Martin–Gruber anastomosis (see Chapter 1).
The medial cord also has a few side branches that can be tested to confirm medial cord involvement. The medial brachial and antebrachial cutaneous nerves originate from the distal medial cord and mediate sensation over the medial aspect of the arm and forearm, respectively. The medial pectoral nerve originates from the proximal medial cord, and if damaged, causes weakness in the sternal head of the pectoralis major.
Posterior Cord: Radial Plus Palsy
The posterior cord is made up of the posterior division of all three trunks, containing input from C5 to C8. The presence of T1 fibers in the posterior cord is controversial and, needless to say, variable. The terminal branches of the posterior cord are the radial and axillary nerves; therefore, a combination palsy of these two nerves is the hallmark of a posterior cord injury (Fig. 5-12). Hence, a posterior cord injury may also be called a radial-axillary palsy or radial plus palsy.
A radial palsy causes weakness in forearm extension (triceps), forearm supination (supinator), wrist extension (extensor carpi radialis longus and brevis, extensor carpi ulnaris), and finger/thumb extension (superficial and deep finger extensors). Radial nerve sensory loss involves the posterior arm (posterior brachial cutaneous nerve) and forearm (posterior antebrachial cutaneous nerve), the lower lateral aspect of the arm (lower lateral brachial cutaneous nerve), and the dorsolateral hand (superficial sensory radial nerve). An axillary palsy causes arm abduction weakness secondary to deltoid paralysis. An axillary nerve lesion can also cause sensory loss in the upper lateral arm (upper lateral brachial cutaneous nerve), especially if the patient is examined soon after injury. Furthermore, arm adduction and internal rotation weakness helps confirm posterior cord damage because the minor branches off the posterior cord control these movements (i.e., the upper and lower subscapular, and thoracodorsal nerves).
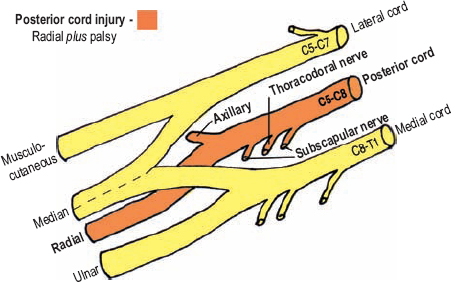
Figure 5-12 Radial plus palsy (radial-axillary palsy, posterior cord injury). The two terminal branches of the posterior cord are the radial and axillary nerves; therefore, a combination palsy of these two nerves is the hallmark of a posterior cord injury.
Summary
To assess the distal brachial plexus, one needs to be familiar with patterns of neurological deficits that may occur following injury to its terminal branches: the musculocutaneous, median, ulnar, radial, and axillary nerves. With this knowledge, diagnosis of cord-level injuries becomes straightforward; cord injuries cause deficits that are a combination of those seen with injury to their branches. Therefore, analogous to proximal brachial plexus injury being assessed by referring to its spinal nerve components, the distal plexus is evaluated by using knowledge of its major terminal branches.
 Assessment of Divisional Injuries
Assessment of Divisional Injuries
Isolated injury to one of the brachial plexus divisions can be difficult to differentiate clinically from cord- or trunk-level lesions. Furthermore, it is usually not possible to say if divisions are damaged in addition to cords or trunks without surgical exploration.
An isolated injury to one, or more, divisions yields neurological deficits that are the same, or less severe, than a cord-level injury. For example, an injury to the anterior division of the lower trunk (C8/T1) should involve nearly all the fibers in the medial cord. However, a divisional injury affecting the lateral cord may only involve the anterior division from the upper trunk (C5/C6) or the anterior division from the middle trunk (C7). Injury to any of the three posterior divisions will cause a partial posterior cord deficit. For example, an isolated injury to the posterior division of the upper trunk may be confused with an axillary nerve palsy; however, presence of some brachioradialis and supinator weakness, along with sensory loss on the dorsal thumb (C6 dermatome via superficial sensory radial nerve) should point to a partial cord or divisional injury rather than an axillary nerve palsy.
With an ability to readily diagnose proximal and distal brachial plexus lesions, one can deduce a divisional injury. Nevertheless divisional lesions may be indistinguishable from partial cord injuries. Fortunately, injuries only involving the divisions are infrequent.
 Diagnosis of Preganglionic Injury
Diagnosis of Preganglionic Injury
Many stretch injuries to the brachial plexus actually cause avulsion of the ventral and dorsal rootlets from the spinal cord itself. The C8 and T1 spinal rootlets are most prone to this type of injury because these spinal nerves have minimal anchoring to their respective troughs along the transverse processes. In contrast, the C5 to C6 spinal nerves are strongly attached to their respective transverse processes, thereby making avulsion at these levels less likely when a similar force is applied. There are multiple ways to determine if a spinal root has a preganglionic, or avulsion, injury, which is very important for prognosis and treatment.
Myelography supplemented with postmyelography CT scanning has good sensitivity in detecting pseudomeningoceles and absent (avulsed) nerve rootlets at each cervical level. These two findings are highly suggestive of rootlet avulsion. Magnetic resonance imaging (MRI) may also be used to document these findings; however, most medical centers do not routinely provide images of adequate resolution. Involvement of very proximal branches of the brachial plexus, including the phrenic nerve (paralyzed diaphragm), dorsal scapular nerve (rhomboid weakness), and long thoracic nerve (scapular winging) also suggests preganglionic damage, or at least intraforaminal damage. Avulsion of the T1 spinal nerve can cause Horner’s syndrome, the presence of which should be documented.
Electrophysiological testing is also quite useful in confirming preganglionic damage. With this type of injury, the peripheral nerve sensory axons remain connected to their cell bodies in the spinal ganglia. Therefore, the amplitudes of distal extremity sensory nerve action potentials (SNAPs) remain normal (or increased), despite no sensation being present on examination. Presence of compound motor action potentials (CMAPs) argue against spinal cord avulsion.
 The Examination Approach
The Examination Approach
The Comprehensive Examination
When examining the brachial plexus and upper extremity, one should employ a comprehensive and systematic approach, beginning proximally and proceeding distally. With the exception of a screening assessment in the emergency department, an abbreviated or focused examination is not recommended. This is because some subtle, yet important, deficits can be missed. My examination routine is divided into six distinct steps (Table 5-1).
• Step 1: The back. The exam begins with the patient facing away from the examiner. The presence of scapular winging, muscle atrophy, and asymmetry of the shoulders and scapulae at rest are noted. Next, the patient shrugs his or her shoulders upward to assess trapezial and levator scapulae function. Having the patient bring the scapulae together assesses the rhomboids. The latissimus dorsi are palpated bilaterally and the patient is asked to cough. The patient is instructed to raise the arms to the sides and above the head to check trapezial function. Next, the patient is told to reach toward the wall with the affected arm and scapular winging is evaluated, first with the arm protracted, and then with it flexed.
• Step 2: The shoulder. Starting with the arm straight and to the side, the patient is instructed to abduct the arm. In doing so, the supraspinatus and deltoid are assessed. With the arm horizontal to the floor, the posterior head of the deltoid (posterior movement) and teres major (downward movement) are tested. The patient then flexes the forearm 90 degrees (“hold-up” position) and both the clavicular (lateral pectoral nerve) and sternal (medial pectoral nerve) heads of the pectoralis major are assessed, both visually and by palpation. Facing the patient’s flank, external arm rotation is tested (infraspinatus).
Table 5-1 The Six Steps of a Comprehensive Brachial Plexus Examination
| 1-Back Observation Rhomboids Latissimusdorsi Trapezius Scapularwinging |
| 2-Shoulder Supraspinatus Deltoid Posterior deltoid Teres major Pectoralis major Infraspinatus |
| 3-Arm Triceps Biceps Brachioradialis |
| 4-Forearm Supinator Pronator Wrist flexion Wrist extension Finger extension |
| 5-Hand Observation Finger flexion Thenar intrinsics Hypothenar intrinsics Interossei Lumbricals |
| 6-Skin Sensation Perspiration/Horner’s Pulses/masses Reflexes/Tinel’s sign |
• Step 3: The arm. The triceps are examined with the upper arm parallel to the floor, so the effect of gravity is eliminated. Forearm flexion at the elbow is tested with the forearm fully supinated (biceps brachii) and half supinated (brachioradialis).
• Step 4: The forearm. Supination and pronation are tested with the elbow straight. Next, wrist movement is assessed. The patient flexes the wrist (flexor carpi radialis and ulnaris), and then the arm is pronated and the forearm extensors are tested (extensor carpi radialis longus and brevis, and extensor carpi ulnaris). With the arm placed on a flat surface, the long forearm finger extensors (extensor digitorum communis, extensor indicis, extensor digiti minimi, and extensors pollicis longus and brevis) are also evaluated.
• Step 5: The hand. The hand is first observed at rest for signs of atrophy. The patient should be instructed to open and close the hand so that any evidence of contracture can be observed, if present (e.g., claw hand). Next, the thumb is evaluated further, including abduction, adduction, opposition, and flexion. The okay and Froment’s signs are checked for. Flexion at the proximal (flexor digitorum superficialis) and distal (flexor digitorum profundus) interphalangeal joints is assessed. Abduction and opposition of the fifth digit is evaluated, as well as Wartenburg’s and palmaris brevis signs. Finger abduction (dorsal interossei), adduction (palmar interossei), and extension at the interphalangeal joints is tested (lumbricals).
• Step 6: The skin. Sensation with light touch and pinprick is tested from the shoulder down to the hand. Testing is performed circumferentially on the arm, forearm, and hand. The fingertips are also tested, which is especially important considering the thumb, long finger, and fifth digit each represent a different dermatome. Any abnormality or asymmetry between the upper extremities is evaluated further, including assessment of two-point discrimination and localization. Lack of sweating may be observed with an ophthalmoscope. The neck and axilla are palpated and observed for scars, masses, or a Tinel’s sign. To finish, pulses and reflexes (C6, C7) are tested.
Localization of the lesion is then performed using one’s knowledge of peripheral nerve anatomy. The deficit may then be graded for both research purposes, as well as for serial assessment of improvement, or lack thereof.
The Screening Examination
In certain situations (e.g., in the emergency department or trauma slot) an abbreviated screening examination for brachial plexus injury may be indicated. This emergent evaluation, however, is often limited by other injuries, including long bone fractures, spine trauma, and patient confusion or stupor. The screening examination, or primary survey, evaluates 9 muscles that have been selected because they each follow a separate path through the brachial plexus (Table 5-2). These muscles include two shoulder, three arm, and four hand muscles.
This primary survey is performed with the patient supine or sitting. The following two shoulder muscles are tested: deltoid, and infraspinatus. Next, the following arm muscles are tested: biceps, brachioradialis, and triceps. Finally, hand movements are tested, including the flexor carpi radialis, extensor indicis, abductor pollicis brevis, and dorsal interossei.
Table 5-2 The Brachial Plexus Screening Examination*
| Shoulder | ||
| Deltoid Infraspinatus | C5, uppertrunk, posterior cord, axillary nerve C5, uppertrunk, suprascapular nerve | |
| Arm | ||
| Biceps Brachioradialis Triceps | C6, uppertrunk, lateral cord, musculocutaneous nerve C6, uppertrunk, posterior cord, radial nerve C7, middle trunk, posterior cord, radial nerve | |
| Hand | ||
| Flexor carpi radialis Extensor indicis Abductor pollicis brevis Dorsal interossei | C7, middle trunk, lateral cord, median nerve C8, lower trunk, posterior cord, radial nerve C8, lower trunk, medial cord, median nerve T1, lowertrunk, medial cord, ulnar nerve | |
| *The 9 muscles are assessed proximally to distally. Spinal nerve contributions are simplified. | ||
If the primary survey reveals a deficit, a secondary survey in the emergency department is performed; this includes sensory and additional motor testing. The primary survey reveals if the lesion is proximal or distal. Proximal lesions are secondarily tested using a spinal nerve template (Which spinal nerves are affected?), whereas distal brachial plexus injuries are assessed with a cord approach (Which cords are affected, and to what extent?). To perform the secondary survey, think of all the muscles innervated by the injured element (as determined by the primary survey, e.g., upper trunk, medial cord) and then test them all from proximal to distal. A comprehensive examination (described previously) should always be performed; however, in most severe trauma patients, this is performed later when the patient is fully cooperative and more time is available.
 Processes Affecting the Brachial Plexus
Processes Affecting the Brachial Plexus
Brachial plexus injuries are most often traumatic, with stretch (including birth palsies), lacerations, gunshots, and blunt contusions being the more common mechanisms. Other, less frequent etiologies include compression by anomalous fibrous ridges on the scalene musculature (neurogenic thoracic outlet syndrome), delayed radiation damage, and acute brachial plexitis (Parsonage–Turner syndrome). Considering the patient’s history and risk factors, an accurate etiological diagnosis can usually be made, even before examination. Some specific causes of brachial plexus injury will be discussed.
Neurogenic Thoracic Outlet Syndrome
Symptoms of neurogenic thoracic outlet syndrome, or Gilliat-Sumner hand, are predominantly caused by irritation to the C8 and T1 spinal nerves, and/or lower trunk. “Disputed,” arterial, and venous forms of thoracic outlet syndrome are separate clinical entities and will not be commented upon. The source of irritation for neurogenic thoracic outlet syndrome is localized to the scalene triangle. The scalene triangle is made up of the anterior scalene anteriorly, the middle scalene posteriorly, and the edge of the first rib inferiorly. The brachial plexus and subclavian artery pass through this triangle, but the subclavian vein does not. Irritation of the brachial plexus is often from an abnormal fibrous band, on or near these two scalene muscles. An elongated C7 transverse process or cervical rib may be present, both of which unfavorably re-orient the borders of the scalene muscles, possibly leading to neural compression or irritation. The patient with classic neurogenic thoracic outlet syndrome has forward drooping shoulders.
Manifestations of neurogenic thoracic outlet syndrome are usually localized to the C8 and T1 nerve roots. These patients present with progressive hand intrinsic weakness and atrophy. Sensory loss, when present, occurs on the medial aspect of the forearm, as well as on the medial third of the hand. In terms of the terminal brachial plexus branches, the hand intrinsic weakness and atrophy are often more median than ulnar, whereas the sensory loss is all ulnar (ulnar and medial antebrachial cutaneous nerves, both from the medial cord). Pain is not usually of significant concern to the patient, but a dull ache in the shoulder girdle or axilla is not uncommon. Externally rotating the arm, while abducting it above the head, often precipitates or worsens symptoms after a minute or two in this position (Roos maneuver or elevated arm stress test [EAST]). Loss of a radial pulse with this maneuver (Adson’s sign) may also occur. Both of these provocative tests, however, have a high rate of false-positives; therefore, they are of limited value. A supraclavicular Tinel’s sign may be present. A cervical radiograph should be ordered to rule out a cervical rib or elongated C7 transverse process.
Care must be taken to differentiate thoracic outlet syndrome from a C8 radiculopathy or ulnar compression at the elbow. Concurrent weakness in median (abductor pollicis brevis) as well as ulnar (abductor digiti minimi or the first dorsal interosseous) innervated muscles helps confirm a more proximal injury at the brachial plexus (i.e., neurogenic thoracic outlet, and not carpal or cubital tunnel syndromes). No history of neck or radicular pain, along with the presence of both C8 and T1 sensory and motor changes, helps exclude a single-level radiculopathy.
Birth-Related Brachial Plexus Palsy
Newborns can have stretch injuries to their proximal brachial plexus during difficult deliveries, vaginal or otherwise. Heavy birth weight is considered a predisposing factor. The most common injury is an Erb’s palsy, where C5 and C6 (or the upper trunk) are damaged. As described previously for adults, an infant with an Erb’s palsy maintains the arm (or arms) adducted, forearm extended and pronated, and wrist flexed (waiter’s tip position). Another type of birth injury is Klumpke’s palsy, with damage to the C8 and T1 nerve roots. Although this palsy can occur during a breech delivery with the arm hyper-abducted above the head, it most commonly occurs after a face-first delivery where the head is hyperextended. Infants with Klumpke’s palsy are not able to grasp with the hand. Other types of birth brachial plexus injury include complete plexal injury and an Erb’s plus palsy. An Erb’s plus palsy is an injury involving C5, C6, plus C7.
Klumpke’s palsy is the least common type of birth palsy. The prognosis of both Klumpke’s palsy and complete brachial plexus palsy are both significantly worse than for Erb’s palsy because the latter often recovers spontaneously in a matter of months.
Examining an infant is challenging. Neurological diagnosis of an obstetrical palsy relies greatly on observing the arm and hand position at rest, its lack of movement, and upper extremity asymmetry during play and crawling. Performing a series of examinations over time is important. Radiographs may be used to rule out fractures or a hemidiaphragm (phrenic palsy). Electrodiagnosis should be first performed 4 to 6 weeks after injury, and then on a serial basis (approximately every 3 months) when indicated to assess recovery. As the child grows, the examination becomes more structured, including various arm movements of functional importance.
Acute Brachial Plexitis (Parsonage–Turner Syndrome)
Acute brachial plexitis presents with marked shoulder pain that often radiates down the arm, up into the neck, or toward the scapula. Its onset is usually sudden and lasts for hours to a few weeks. This syndrome occurs more often in males and can affect any age group. A common position of comfort is the arm adducted and forearm flexed, the so-called adduction-flexion sign of acute brachial plexitis. Neck movement and Valsalva maneuvers do not exacerbate the pain. Cervical imaging helps rule out radiculopathy from a herniated disc. Weakness is usually not present during the acute, painful phase of this syndrome; however, as the pain resolves, paralysis of certain brachial plexus innervated musculature occurs. The degree of eventual weakness often correlates with the severity of the initial pain. Although any, or all, upper extremity and shoulder girdle muscles may be involved, the most common muscles affected include the deltoid, supraspinatus, and infraspinatus. This syndrome may occur bilaterally, especially subclinically (i.e., seen only with electrodiagnostic testing). Sensory loss is classically absent or minimal during acute brachial plexitis, a finding that helps confirm the diagnosis. Because it is a benign and self-limiting process, approximately 90% of patients return to near normal in up to 3 years. The etiology of acute brachial plexitis is uncertain. About half of the patients report an antecedent, usually a viral illness, which points toward an inflammatory cause. Some patients report mild-to-moderate shoulder trauma or overuse as an instigating factor. Others may have recently undergone a surgical procedure. Electrophysiology is helpful for both diagnosis and prognosis.
 A rare autosomal dominant form of acute brachial plexitis has been reported. These patients have repeated bouts of characteristic pain and weakness, often involving different nerves. Although these episodes often follow stressful events, including childbirth, strenuous exercise, and infection, the majority of cases have no identifiable cause. This hereditary disease can also affect children.
A rare autosomal dominant form of acute brachial plexitis has been reported. These patients have repeated bouts of characteristic pain and weakness, often involving different nerves. Although these episodes often follow stressful events, including childbirth, strenuous exercise, and infection, the majority of cases have no identifiable cause. This hereditary disease can also affect children.
Radiation-induced Brachial Plexopathy
Plexitis may occur following radiation treatment of regional cancers, usually breast cancer. Radiation damage can occur at any time after exposure (on average 5 years), and usually causes painless sensory and motor deficits in either the upper trunk or entire plexus; sole involvement of the lower trunk is uncommon. Horner’s syndrome usually does not occur, and if it does, metastatic plexopathy or an apical lung metastasis should be investigated. Local radiation-induced skin changes are a common finding in these patients, although not universal. (See also the comments on radiation-induced plexitis in the lumbosacral plexus section.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





