The Cognitive Executive Is Implicated in the Maintenance of Psychogenic Movement Disorders
Sean A. Spence
ABSTRACT
Psychogenic movement disorders (PMDs) comprise a range of functional abnormalities characterized by motor inconsistencies and differentiated, diagnostically, by varying degrees of inferred conscious causation (on the part of the patient). At one extreme is conversion disorder (“hysteria”), in which patients are thought to be unaware of the mechanism of their impairments; at the other extreme is feigning (malingering), in which patients are suspected of consciously, deliberately, deceiving the physician. These alternatives are difficult to differentiate reliably and, to be valid, their diagnoses would require that the physician either know what the patient is thinking or else demonstrate some pathognomonic physical sign or investigative finding (currently lacking). The characteristic physical features of PMD tend to be those of inconsistent motor dysfunction, which may remit with distraction or sedation. Though these features may help to distinguish PMD from organic pathology, they are less helpful in distinguishing conversion from feigning. Here, we consider the evidence that subcategories of PMD share the following feature: reliance upon the cognitive executive. The executive comprises those control processes engaged in the production of nonroutine, nonautomated behaviors—behaviors that require attention to action. It is because executive resources are constrained that they may decompensate with sufficient distraction or sedation, thereby revealing a “normal” motor system. In this regard, PMDs behave differently to most exemplars of organic movement disorder.
INTRODUCTION
Psychogenic movement disorders (PMDs) present obvious difficulties for the clinician. The absence of demonstrable organic pathology is accompanied by inconsistent motor signs, and although the operations of the motor system manifesting disorder are those over which a conscious
agent has a degree of control, the patient who expresses these abnormalities denies producing them (consciously, at least). Such a situation may be difficult to manage clinically. The physician wishes to be certain that no organic pathology has been missed, thereby risking overinvestigation. The patients wish to establish the veracity of their symptoms, thereby risking exaggeration. They may swap one doctor for another, transferring their care at the very point at which continuity is most crucial—when physical investigations have excluded organic pathology. Failure to obtain appropriate psychiatric evaluation at this point may lead to further unnecessary (and perhaps even dangerous) investigations. In the background, impending litigation may complicate matters: the patient perhaps feels wronged because of a causal injury sustained at work, while the physician may be sensitive to the possibility of being duped for financial gain.
agent has a degree of control, the patient who expresses these abnormalities denies producing them (consciously, at least). Such a situation may be difficult to manage clinically. The physician wishes to be certain that no organic pathology has been missed, thereby risking overinvestigation. The patients wish to establish the veracity of their symptoms, thereby risking exaggeration. They may swap one doctor for another, transferring their care at the very point at which continuity is most crucial—when physical investigations have excluded organic pathology. Failure to obtain appropriate psychiatric evaluation at this point may lead to further unnecessary (and perhaps even dangerous) investigations. In the background, impending litigation may complicate matters: the patient perhaps feels wronged because of a causal injury sustained at work, while the physician may be sensitive to the possibility of being duped for financial gain.
Likewise, there are conceptual problems. The diagnosis of conversion disorder relies upon Freudian notions of the psychodynamic unconscious, which have been superseded in some contemporary psychoanalytic accounts of hysteria (1, 2, 3). Clinically, physicians are required to infer unconscious processes in the patient, which are essentially theoretic abstractions. They are hard to demonstrate reliably. There is also ample risk of dualism: splitting the mind (psychogenic) from the body (organic, “real” disease); the conscious (feigning) from the unconscious (conversion); the dishonest from the sincere. It is all too easy for the patient to be construed as either a victim or a villain, hysteric or deceiver on the basis of inferences drawn from inconsistent physical findings (4).
In this chapter, we adopt an agnostic position on the differentiation between conversion disorder and feigned symptomatology, recognizing that clinically there is little which might objectively sustain such a distinction (4,5). Instead, we shall regard PMD as a heterogeneous entity, which nevertheless exhibits certain unifying features. We will argue that PMD is differentiated from organic movement disorders by the way in which it relies upon the engagement of the cognitive executive for the maintenance of its clinical expression (motor phenomenology).
COGNITIVE ARCHITECTURE: THE ROLE OF THE EXECUTIVE
In cognitive neuropsychological accounts of brain function, a distinction is often made between processes (and behaviors) that occur automatically and those that require the intervention of higher centers or systems, to generate novelty or complexity. Examples of the former may be drawn from everyday life, in which much of our behavior may be relatively overlearned, routine, and automated, requiring very little of our conscious attention, for example, the movements we execute while driving a car. We can attend to these movements (if necessary), but in general, because their demands are low, we are able to attend to other behaviors as well, for example, conversing while driving. However, when the task demands change or a nonroutine occurrence intervenes, we are able to redirect our attention to the task in hand, for example, we change our motor routines when we realize that there is fog on the road ahead. The motor behaviors that we have automated through overuse may be inappropriate in certain new situations. For instance, if I am used to driving along a particular route to work and have to travel part of that same journey on an errand on the weekend, I may, if distracted, continue driving toward my place of work instead of turning off onto the new route. In this context, my routine behavior has become inappropriate. It has intruded into the new motor program.
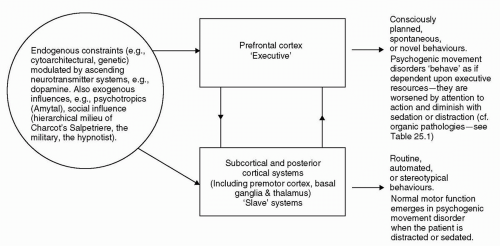
A number of authors have conceptualized cognitive architectures that might support such changes in emergent behavior (6, 7, 8, 9). Commonly, these architectures privilege the prefrontal cortex, with it serving to modulate lower, “slave” systems within the brain (Fig. 25.1). Within such a model, the premotor, motor, and sensory cortices, together with specific subcortical regions (such as the basal ganglia and cerebellum), are hypothesized to be sufficient for the performance of routine cognitive/motor procedures. Much automated behavior can be conceptualized as programs run on such subordinate brain systems. However, in nonroutine situations, the intervention of prefrontal systems is required. Hence, the prefrontal cortex is involved in such executive tasks as planning, selecting, initiating, and inhibiting behavior; the programming and execution of such behaviors being delegated to posterior, lower systems (10,11).
An example of this cognitive architecture is provided by a simple protocol that we have used to study the functional anatomy of mouth movements (12). We utilized positron emission tomography (PET) to study healthy subjects under three conditions:
Rest: Lying in the scanner listening to a pacing tone emitted by a computer metronome at a rate of one tone every 3 seconds (0.33 Hz).
Stereotypic responding, in which subjects responded with one vocal articulation each time they heard the pacing tone (0.33 Hz), specifically enunciating the following repetitive sequence: “Lah, bah, bah, lah, lah, bah, bah, lah …,” one syllable at a time.
Freely selected responding, in which subjects responded to each tone (0.33 Hz) by saying either “lah” or “bah” in a sequence of their own choosing, the instruction being that they make the sequence as random as possible.
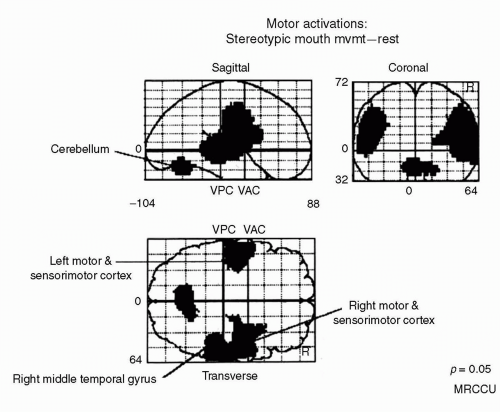
The stereotypic response condition requires that subjects perform behaviors that are totally determined by the experimenter. In response to a tone, the subjects have only one response to make that is appropriate (lah or bah,
depending upon their progress through the stereotypic sequence). When we compared brain activity during this condition with that of the resting state, we found bilateral premotor and motor cortex activation, together with cerebellar activity (Fig. 25.2). Hence, the motor system was activated during performance of the task, but there was no significant activation in prefrontal regions. Lower brain areas were sufficient to produce stereotypic responding.
depending upon their progress through the stereotypic sequence). When we compared brain activity during this condition with that of the resting state, we found bilateral premotor and motor cortex activation, together with cerebellar activity (Fig. 25.2). Hence, the motor system was activated during performance of the task, but there was no significant activation in prefrontal regions. Lower brain areas were sufficient to produce stereotypic responding.
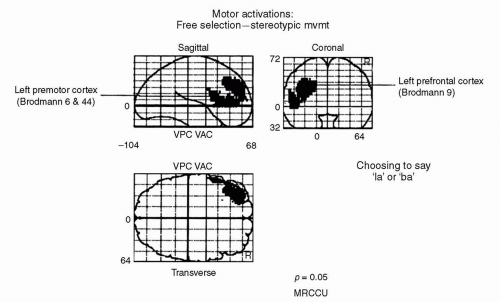
When subjects were allowed a degree of choice in their response, albeit constrained to two possible exemplars (lah or bah), there was increased activation in left prefrontal cortex. Figure 25.3 shows those brain regions exhibiting greater activation during freely selected response compared with the stereotypic condition (12).
Hence, when there is choice over the selection of motor response, there is greater activation in prefrontal cortex (particularly left dorsolateral prefrontal cortex, DLPFC) (11,12). Similar results have been found when subjects choose which finger to move (13) or which direction to move a joystick (12). The commonality among these findings is that prefrontal activation is necessary when subjects are called upon to choose their response to the environment. Subordinate brain regions (e.g., premotor and motor cortices) are no longer sufficient for the production of such behavior.
PROBING THE EXECUTIVE
In the clinical setting, we are often concerned with assessing the cognitive executive. Implicit in such assessment is the acknowledgement that the executive is central to independent living, its dysfunction impacting upon patients’ day-to-day lives (14).
Part of the mental state examination used by psychiatrists comprises the performance of orthographic verbal fluency. The patient is asked to produce a sequence of verbal responses on the basis of an arbitrary letter rule, for example, “say as many words as you can in the next minute, beginning with the letter ‘s’” (excluding proper nouns and numbers). These sorts of tasks are used to probe prefrontal function. Subjects are allowed to generate self-selected responses, although they are, of course, constrained to selecting them from a specified category of exemplars (e.g., words beginning with the letter “s”). When verbal fluency has been studied using functional neuroimaging [PET or functional magnetic resonance imaging (fMRI)], it has been shown to activate left DLPFC and anterior cingulate cortex (13,15,16). Such findings are congruent with a neuropsychiatric literature implicating lesions or functional abnormalities of the left DLPFC in expressive and dynamic, or transcortical motor, aphasia (17) and the alogia seen in schizophrenia and depression (18).
However, healthy subjects may also be made to exhibit decrements in executive performance if cognitive demands are increased sufficiently. Hence, if subjects perform the verbal fluency task while also attempting to generate random sequences of movements with a joystick, the randomness of the latter will be less than when they perform such movements alone (19). Therefore, having to generate self-chosen words at the same time as self-chosen movements (dual
tasking) impairs the subject’s ability to generate novelty. The normal executive system is subject to demonstrable processing constraints (20). (Various authors have demonstrated that the threshold for such decompensation may be lower in neuropsychiatric disorders.) (6)
tasking) impairs the subject’s ability to generate novelty. The normal executive system is subject to demonstrable processing constraints (20). (Various authors have demonstrated that the threshold for such decompensation may be lower in neuropsychiatric disorders.) (6)
This principle is borne out in another context when healthy subjects generate a sequence of single responses under increasing task demands. If subjects are asked to respond faster, then there is a demonstrable fall off in the degree of randomness they can generate (i.e., their numeric responses become more stereotypic) (6). Similarly, if the set of possible response exemplars is increased, for example, from digits between 1 and 4 to digits between 1 and 32, then the subjects require longer to respond. Hence, the cognitive substrate of their ability to generate random response sequences is doubly constrained by processing speed and capacity. If time to respond decreases, then performance diminishes. If cognitive load (the set of exemplars) increases, then processing time increases. The cognitive executive is constrained by finite resources, and its constraint is manifest in performance decrements (6).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree