9 The cortex
Embryological development
Neuronal fate in the mammalian cortex is influenced by the timing of cell differentiation, which is dependent on both genetic and environmental factors (see Chapter 2). The cerebral cortex neurons are generated in the ventricular zone by the epithelial layer of progenitor cells that line the lateral ventricles. They migrate to the cortical plate, which eventually develops into the grey matter of the cortex. The final position assumed by these neurons depends on their ’birthmoment’, or time of last division. The migration occurs along radially organised glial cells called radial glia, which guide the migrating neurons to the cortex. The layering of the neurons in the cerebral cortex is established with an inside-first, outside-last manner so that the newest neurons must pass over and around the more mature neurons, probably gaining information from the previously established neurons as they pass.
The meninges
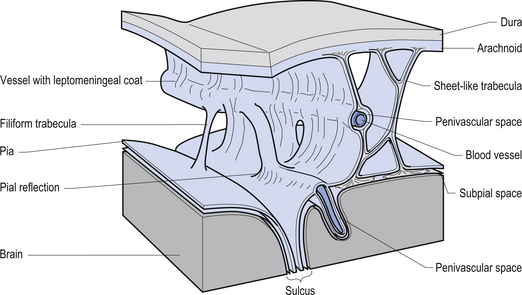
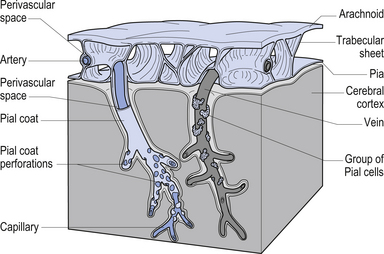
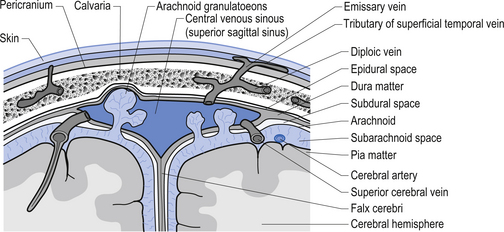
The meninges are layered structures that contain cerebrospinal fluid and give protection to the brain and spinal cord. The meninges are composed of three layers; the dura mater, the arachnoid mater, and the pia mater (Figs 9.1 and 9.2).
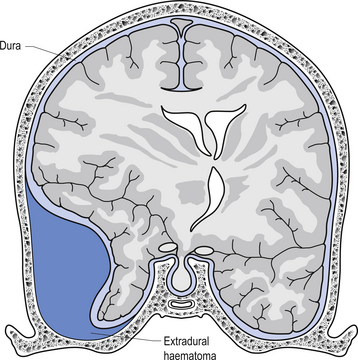
The way in which the meninges connect to each other and the structures that they attach to gives rise to three clinically important spaces or potential spaces, the epidural space, the subarachnoid space, and the subdural space. The epidural space is a potential space that can form between the dura and the bone of the skull. The meningeal arteries run in the space between the tightly adherent dura and the skull. The middle meningeal artery passes under and along the temporal bone, which is the thinnest bone of the skull and thus the most easily fractured. Trauma to the temporal bone can cause tears in the meningeal arteries and result in blood escaping into the potential epidural space. As the blood builds up, it forces the periosteal layer of dura away from the bone and bulges into the arachnoid and pia layers, eventually exerting pressure on the brain. This process is referred to as an epidural haematoma (Fig. 9.3).
Epidural haematomas are usually rapidly growing and expanding as the arterial pressure spreads the periosteal dura from the bone of the skull. The dural separation continues until it reaches a cranial suture where the dura is much more tightly joined to the skull. This results in an expansile lesion that takes the shape of a biconcave lens. Clinically, the patient may experience a lucid interval following trauma to the skull where they may not have any symptoms. Within a few hours the expanding haematoma starts to compress the brain and results in increased intracranial pressure and death if not treated.
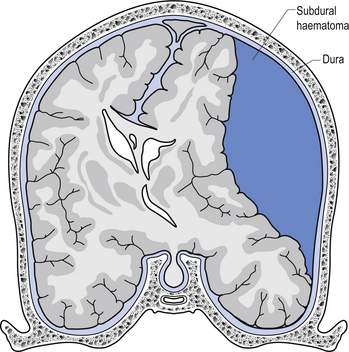
The symptoms of SDH often mimic other cerebrovascular events or space-occupying lesions. Alcohol consumption reduces clotting mechanisms and often results in head trauma from falls. Anticoagulants can also increase the risk of SDH from minor trauma in the elderly (Fig. 9.4). Chronic subdural haematomas can take weeks to months in the elderly before they start to experience symptoms. This is mainly due to the low-pressure, slow leak from the veins and the fact that brain tissue shrinks somewhat as we age and allows a greater space for the blood to occupy before interference with function occurs. Acute subdural haematomas require a considerable amount of traumatic force to occur and as such are usually associated with other serious brain injuries such as traumatic subarachnoid haemorrhage and brain contusions.
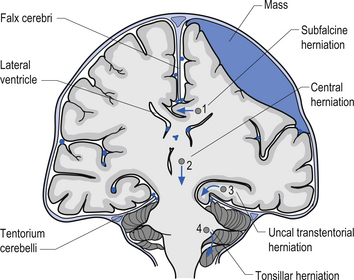
1. Transtentorial herniation involves the compression and protrusion of the medial temporal lobe, usually the uncus or periuncal areas, inferiorly through the tentorial notch (Fig. 9.5). Because of the pressure exerted on the mesencephalic area and the peduncles of the cerebrum, oculomotor dysfunction and hemiplegia can result. The oculomotor damage usually results in a dilated pupil ipsilateral to the side of the lesion due to the unopposed action of the sympathetic nerves. The hemiplegia usually occurs on the side opposite the lesion. Damage to the mesencephalic reticular system can also lead to loss of consciousness and coma.
2. Central herniation is the downward displacement of the brainstem. The action of the downward displacement may traction the abducens nerve (CN VI) and cause lateral rectus palsy (Fig. 9.5).
3. Tonsillar herniation occurs when increased intracranial pressure forces the tonsillar region of the cerebellum down through the foramen magnum of the skull. Because of the high pressure experienced in the medullary region, this condition usually results in respiratory arrest, blood pressure instability, and death (Fig. 9.5).
4. Subfalcine herniation results when the cingulate gyrus is pushed under the falx cerebri and into the other half of the brain. No specific clinical signs may be present with this condition (Fig. 9.5).
Cerebral spinal fluid (CSF)
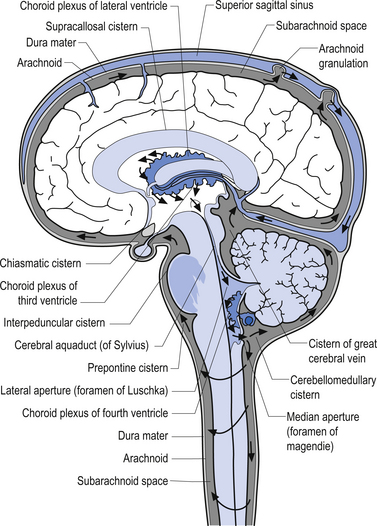
Cerebral spinal fluid is normally a clear, colourless, and odourless fluid that diffuses over the brain and spinal cord. CSF probably functions to cushion the brain and spinal cord from external jarring or shocking forces that may be transmitted through the tissues to reach these structures. CSF may also function in some capacity as a metabolic transport medium, transporting nutrients to the neuraxial cells and metabolic waste products away from the neuraxial components. CSF may also function as a pressure distributor in cases where changes in intracranial volume have occurred such as in postoperative lesions where the removed tissue area fills with CFS. The CSF is formed by the dialysis of blood across the tissues of the choroid plexuses found in the ventricle of the brain and brainstem. The circulation of CSF occurs in two systems: the internal system which includes the two lateral ventricles, the interventricular foramens, the third ventricle, the cerebral aqueduct, and the fourth ventricle; and the external system, which includes all of the external spaces surrounding the brain and spinal cord including the various cisterns. Communication between the internal and external systems occurs via two lateral apertures in the fourth ventricle referred to as the foramens of Luschka, and a medial aperture also in the fourth ventricle referred to as the foramen of Magendie. The total volume of CSF in all systems measures about 150 cm3. The CSF is formed at the rate of about 20 cm3/hr or about 480 cm3/day. This means that all of the CSF in your body is replaced about 3.2 times per day. This is accomplished via absorption of the CFS by the arachnoid granulations located along the superior longitudinal sinus which allow the CSF to enter the venous drainage system and return to the general circulation (Fig. 9.6). The CSF circulates from the lateral ventricles, through the third ventricle, to the fourth ventricle where it then enters the external system and bathes the spinal cord and the external surface of the brain (Fig. 9.7).
Summary of CSF examination
| Finding | Suspected condition |
|---|---|
| Neutrophils and decreased glucose | Acute bacterial meningitis |
| Neutrophils and normal glucose | Brain abscess |
| Lymphocytes and decreased glucose | Virus, tuberculosis, or cryptococcal |
| Lymphocytes and normal glucose | Virus, brain tumour, syphilis |
Clinical examination of cerebral spinal fluid
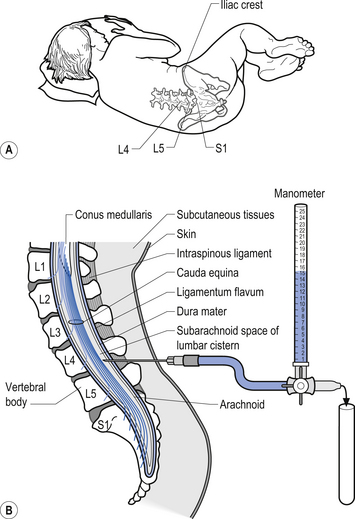
Examination of the CSF can be a valuable tool in the diagnosis of several conditions such as infection that can affect the neuraxis. A common procedure utilised to obtain CSF is the lumbar puncture. This procedure provides direct access to the subarachnoid space of the lumbar cistern which contains the CSF (Fig. 9.8). This procedure can be used to obtain samples of CSF, measure the pressure of the CSF, remove excess CSF if necessary, and act as a conduit for the administration of medication or radiographic contrast material. The CSF is examined for a variety of different elements:
1. CSF pressure—Normal value is 100–200 mmH2O (7.7–15.4 mmHg). Elevated CSF pressure may be caused by blockage of the ventricular drainage system, overproduction, or space-occupying lesions. The two most common causes are meningitis and subarachnoid haemorrhage. Brian tumours and abscesses will cause an increase after a delay of days to weeks.
2. CSF appearance—Normal CSF is clear and colourless. CSF is generally white or cloudy if significant white blood cells (WBC) are present (over 400/mm3). CSF may appear red or pink if red blood cells (RBC) are present; however, if RBC have been in the CSF for more than 4 hours the fluid may appear yellow (xanthochromia). This is due to the breakdown of haemoglobin.
3. CSF glucose—Normal is 45 mg/100 mL or higher. The most significant clinical finding is a decrease in glucose concentration. This occurs in virtually every case of bacterial meningitis. Other causes of decreased glucose include:
4. CSF protein—Normal value is considered to be 15–45 mg/100 mL in adults. In newborns it may range as high as 150 mg/100 mL. The most significant clinical finding is an elevation of protein concentration. Causes of increased protein concentration include:
5. CSF cell count—Normally the CSF contains no more than 5 cells/mm3. Under normal conditions virtually all of the cells present should be lymphocytes. Usually the highest leukocyte counts are found in acute bacterial infections such as meningitis. Usually the cell type most contributing to the leukocytosis in bacterial infections is the polymorphonuclear cell or neutrophil.
Clinical syndromes involving the meninges
Cerebral abscesses
1. Resulting from a contiguous site of focal infection, i.e. dental infection, sinusitis, otitis;
2. Resulting from a distant site, usually haematogenous spread from a lung infection; and
The classic triad of headache, fever, and focal nerve deficit is present in about 50% of cases. Other symptoms include seizures, papilloedema, nausea and vomiting, and nuchal rigidity (Vogel 1994).
Meningitis
Once a pathogen gains entrance to the CSF, the immune system’s counterattack is severely hampered, until a substantial population of the pathogen stimulates neutrophilic pleocytosis in the case of bacteria invasion or lymphocytic pleocytosis in the case of a virus invasion (Scheld 1994; Pryor 1995). Bacteria gain entrance to the CSF by one of three proposed mechanisms:
1. Chemicals released from the bacteria cause relaxation of the intercellular tight junction between the cells forming the blood–brain barrier.
2. Bacteria enter through the fenestrations of the choroid plexus.
3. Bacteria enter inside of macrophages or other cells normally circulating through the CNS.
The primary results of bacterial invasion are:
1. CSF neutrophilic pleocytosis;
2. Increased permeability of the choroid plexus leading to increased CSF pressure, which results in an increased intracranial pressure;
3. Decreased cerebral blood flow;
Normal concentration of WBC in the CSF is 0–5 cells/mm3, and almost all are normally lymphocytes.
Meningitis may be caused by a variety of organisms, including Cryptococcus neoformans, which is a yeast found in the soil with a worldwide distribution. The incidence is about 5/1 000 000 and it is most often found in people with a compromised immune system. Risk factors of immune compromise include lymphoma, diabetes, and AIDS. Symptoms of cryptococcal meningitis include:
Treponema pallidum (Syphilis)
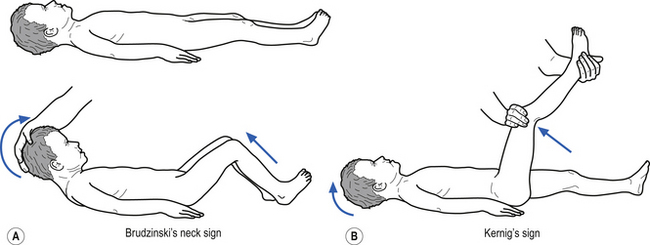
• Stiff neck (Kernig’s and Brudzinski’s signs may be positive –Fig. 9.9);
• Stiffness of shoulders and arms;
• Mental status changes including confusion, disorientation, decreased attention, irritability, sleepiness, and lethargy;
Treatment
Treatment goals are to cure the infection, reduce progression of the disorder, and reduce nerve damage as any existing nerve damage is permanent. Penicillin, tetracycline, and erythromycin are the drugs of choice. Dramatic improvement of symptoms may occur after treatment. However, a progressive disability may result.
Haemophilus influenzae
• Irritability, poor feeding in infants;
• Below normal temperature in young infants;
• Severe headache (loud screeching scream);
• Stiff neck or crying when neck flexed (Kernig’s and Brudzinski’s may be positive – Fig. 9.9);
• Pain in the back when neck is flexed or chin brought to chest;
• Bulging of the fontanelles (soft spots in the skull) of infants;
• Opisthotonos (lying with the back arched, head back, and chin up);
Meningococcus
Summary of causative agents
| Age | Agent |
|---|---|
| Less than 1 month | Group B strep. |
| 1 month to 5 years | H. Influenzae |
| 5–29 years | N. meningitidis |
| Over 29 years | S. pneumoniae |
Staphylococcus (aureus, epidermidis)
This condition is usually caused by the bacteria Staphylococcus aureus or Staphylococcus epidermidis. It may develop as a complication from surgery or from haematogenous spread from another site. Risk factors include brain surgery, CSF shunts, infections of the heart valves, and previous brain infections such as an abscess or encephalitis. The symptoms include:
CSF and serum cultures may show staph, and infections of this type often result in death.
Migraine
Some evidence suggests that pain-sensitive dura and middle meningeal artery wall may contribute to the pain of migraine headaches (see Chapter 17).
Vascular accidents
Arteriovenous malformation (AVM)
These are congenital malformations of the arteriovenous junctions that result in large tangled areas that are often structurally delicate and can be ruptured with relatively minor trauma. The haemorrhage occurs in sinusoidal vessels that are under low pressure. These types of haemorrhage often result in focal neurological signs and headache, epilepsy, and occasionally hydrocephalus. The arachnoid villi can become blocked by blood from repeated subarachnoid haemorrhages and therefore impair CSF resorption, which leads to hydrocephalus.