, Nima Etminan1 and Daniel Hänggi1, 2
(1)
Neurochirurgische Klinik, Universitätsklinikum Düsseldorf, Düsseldorf, Germany
(2)
Medical Art Christine Opfermann-Rüngeler, Zentrum für Anatomie Heinrich Heine Universität, Düsseldorf, Germany
The two burning questions regarding the management of cerebral aneurysms currently concern whether to use clip or coil and what to do with incidental aneurysms. Further issues that require continuing attention and research are primary and secondary damage following subarachnoid hemorrhage (i.e., delayed cerebral ischemia) and perhaps primary prevention of cerebral aneurysm formation by pharmaceutical treatment.
1.1 Clip or Coil?
Microsurgical treatment has become a second-choice method of care for intracranial aneurysms that appear to be not easily amenable to endovascular therapy [1]. A neurosurgical service not providing both treatment modalities can no longer offer competent care of patients with ruptured or unruptured aneurysms.
In many clinical situations, there is insufficient evidence for a clear decision with regard to the optimum mode of treatment. There is usually a gray zone where arguments for both options can be found. It is therefore an accepted requirement that the neurosurgeon and the endovascular therapist decide together on the basis of the patient’s clinical condition, the configuration of the aneurysm as shown on digital subtraction angiography and CT or MR angiography, and their personal experience. Location is an important factor for the decision. Aneurysms of the basilar artery are clearly the domain of endovascular management, for which neurosurgical services do not have the necessary proficiency, but peripheral aneurysms of all vascular territories are still the domain of microsurgery in many centers. Aneurysms of the main bifurcation of the middle cerebral artery (MCA) are within a gray zone, for which there are arguments and data favoring either treatment modality.
Aneurysm size and neck configuration should not play a significant role in the treatment decision. Larger aneurysms and broad-based aneurysms are more difficult for both endovascular and microsurgical techniques, whereas small or narrow-necked aneurysms are simpler and safer to treat with both methods.
It would not be realistic to negate treatment logistics in choosing the treatment modality. If the experience of the available endovascular or microsurgical partner clearly dominates, this factor should be considered.
A more difficult question is the value of the patient’s wishes in the situation of acute subarachnoid hemorrhage. Patients are often cognitively impaired after acute subarachnoid hemorrhage, and they have limited access to scientific knowledge and to second opinions, because treatment decisions must be made under time pressure. Last but not least, the process of informing the patient must be done cautiously, without causing additional stress, in order not to provoke aneurysm rerupture. In the situation of subarachnoid hemorrhage, therefore, the therapists usually decide on the treatment modality, taking account of any preferences clearly stated by the patient. After the decision, the recommendation is transmitted to the patient with a degree of detailed information appropriate to his or her condition.
1.2 To Treat or Not to Treat Incidental Aneurysms?
The principal treatment indication is rarely in question with ruptured aneurysms, apart from some World Federation of Neurosurgical Societies (WFNS) grade 5 hemorrhages with severe early brain injury and uncontrollable intracranial pressure [2]. In view of the high prevalence of unruptured intracranial aneurysms (UIAs) in the general population (2–3 %) and the fact that only a fraction of these rupture during the lifetime of the individual harboring the UIA, the appropriate assessment of an indication for treatment of an incidental aneurysm is somewhat more challenging [3]. A number of studies give us some estimate of the natural history of incidental aneurysms, the specific risk factors for aneurysm rupture, and the risk of morbidity and mortality associated with treatment [4–8]. However, most studies on the natural history of UIAs have been partially biased through specific selection of subgroups, so that controversy continues regarding the true natural history of UIAs [9, 10]. Nevertheless, aneurysm size and location can be considered the major risk factors for rupture of incidental aneurysms. Here, the data from the International Study of Unruptured Intracranial Aneurysms (ISUIA) [8] and the Unruptured Cerebral Aneurysm Study of Japan (UCAS Japan) [7] have generally suggested a low 5-year risk of rupture (1–2 %) for incidental aneurysms with a maximum diameter below 7 mm. Interestingly, the more recent data from the UCAS Japan study [7] suggested distinctly higher rupture risks for aneurysms of the anterior or posterior communicating artery and of lobulated shape, compared with other aneurysms of the anterior circulation and those of nonlobulated morphology. Risk factors of second priority include previous aneurysmal subarachnoid hemorrhage, family history of UIAs or subarachnoid hemorrhage, Japanese or Finnish ethnicity, untreated hypertension, active smoking, and associated collagen diseases.
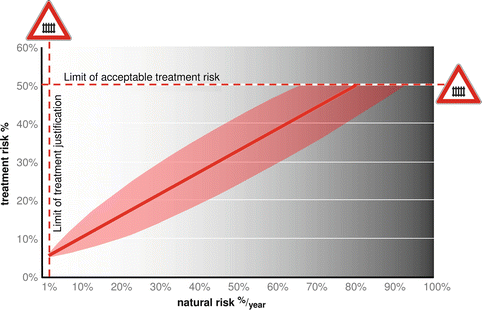
Although aneurysm size is a major risk factor for rupture, it is also the main risk factor (in addition to patient age) for treatment complications and morbidity. Pooled data from a meta-analysis of surgical and endovascular repair of UIAs suggest an overall 6–7 % risk of permanent morbidity or mortality [6, 11]. In detail, the treatment risks for small aneurysms of the anterior circulation amount to approximately 1 % per mm in diameter for both treatment modalities. The risk of endovascular obliteration in the posterior circulation appeared comparable to the risk for the anterior circulation, but the complications for microsurgery were twice as high in the ISUIA data set: that is, the risk of poor outcome 1 year after microsurgical obliteration amounted to some 50 % for giant posterior circulation aneurysms [8]. Ultimately, the assessment of patients with UIAs should be multifactorial and ideally should weigh the estimated risk of rupture against the risk of treatment in a patient with a UIA (Fig. 1.1). Importantly, this assessment should account for the fact that the lifetime risk of rupture, especially in patients younger than 40 years or in patients with the aforementioned risk factors, may be stochastic and thus difficult to estimate based on the currently available data.